- Physiology of the cardiovascular system
- Blood pressure
- Hypertension
- Congenital heart disease
- Investigations
- Cyanotic heart disease
- Congestive heart failure
- Left-to-right shunts
- Obstructive lesions
- Dysrhythmias
- Circulatory maladaptation at birth
Introduction
Congenital heart disease is one the most common significant abnormalities in the newborn and acquired cardiovascular problems are common in preterm or sick infants in the neonatal intensive care unit (NICU). A thorough understanding of the presentation and management of the common cardiovascular disorders is therefore important. This chapter focuses on the medical management of common disorders. Surgical management is not discussed as this generally takes place after the neonatal period. Nursing management is discussed thoroughly in Nursing the Neonate.
 Link to Nursing the Neonate: Nursing newborn babies with congenital heart disease. Chapter 11.
Link to Nursing the Neonate: Nursing newborn babies with congenital heart disease. Chapter 11.Physiology of the Cardiovascular System
The cardiovascular system undergoes major changes in the hours and days after birth. The transition of the circulation from fetal to neonatal is described in Chapter 1. Failure of organ perfusion is a major component of many neonatal disorders, and an understanding of cardiovascular physiology is important in analysing the most appropriate management strategies.
Cardiac Output
Cardiac output (CO) is the volume of blood ejected from the heart per minute. In the neonatal period pulmonary vascular resistance falls rapidly after birth, with a consequent reduction of right ventricular afterload, whereas systemic vascular resistance gradually increases, resulting in an increasing left ventricular afterload; this leads to a doubling of left ventricular stroke volume with no significant change in right ventricular stroke volume. This means cardiac output increases after birth with cardiac performance near the upper limit of its range. Cardiac output (mL/min) is defined as stroke volume (mL/beat) times heart rate (bpm):
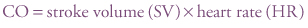
Cardiac output can only be measured directly using an invasive pulmonary artery catheter. Proxy measures use echocardiography to measure the velocity of flow through the aortic valve in conjunction with the diameter of the valve. Ultrasound cardiac output monitors are now becoming available.
Stroke Volume
Stroke volume (volume ejected per heart beat) is a complicated function, dependent on the stretch undergone by individual heart myofibrils.

- Preload. This represents the passive stretching of the resting heart (end diastole), and is largely influenced by venous return and hence vascular volume. Starling’s law states that stroke volume increases with increasing end-diastolic volume until a maximum myofibril stretch is reached. Underfilling (reduced preload) or overfilling (increased preload) causes contraction to be less efficient.
- Afterload. This is the resistance to ventricular contraction distal to the ventricles. A variety of factors are involved, including peripheral vascular resistance and the viscosity of the blood.
- Contractility. This refers to the metabolic state of the heart muscle itself and is largely independent of both preload and afterload.
Cardiac output can be increased by increasing myocardial contractility (inotropy) or increasing the heart rate (chronotropy). These changes are mediated through α, β or dopaminergic adrenergic receptors. These effects are summarized in Table 16.1.
Table 16.1 Adrenergic receptors and effect of stimulation
| Receptor | Effect |
| β1 | Increases myocardial contractility and heart rate |
| β2 | Increases pulmonary and systemic vasodilatation |
| α1 | Causes arteriolar constriction (vasoconstriction) |
| Dopaminergic | Vasodilatation in vascular beds such as the kidney, brain and gut |
Blood Pressure
Blood pressure (BP) is important to maintain organ perfusion. It is defined as the product of flow and resistance according to the formula:
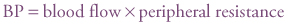
Normal Range
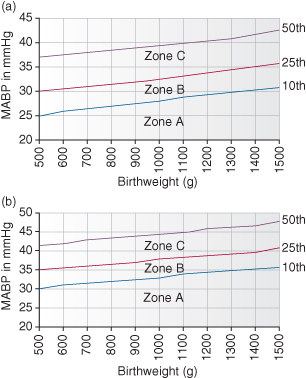
Blood pressure (BP) normally varies with gestational age and postnatal age, and both need to be considered when deciding whether a baby needs treatment for low BP. The normal range against birthweight is shown in Fig. 16.1.
Figure 16.1 (a) Range of mean arterial blood pressure (MABP) measurements in very low birthweight infants less than 36 h of age and (b) more than 36 h old. Zone A: Hypotension requiring treatment (see Fig. 16.2). Zone B: Possible hypotension requiring clinical assessment. Zone C: BP unlikely to require treatment but clinical signs should be assessed.
Hypotension
Hypotension is a common and important complication of the sick newborn infant, but BP is not in itself the critical measure in which the clinician is interested. Tissue perfusion is essential, because once this falls below a critical limit, organ function will fail. The clinical measurement of blood flow and vascular resistance is not possible, and so BP is the physiological measurement that is used instead; see Box 16.1.
- Metabolic acidosis. Tissue hypoxia leads to anaerobic metabolism and lactate production
- Reduced urine output. Failure to perfuse kidneys leads to oliguria or eventually anuria
- Poor skin perfusion. Capillary refill time (normally ≤2 s) is a good way to assess the circulation. Prolonged capillary refill may indicate shock. Mottling of the skin (cutis marmorata) occurs with prolonged underperfusion
- Reduced conscious level. With sever circulatory failure brain perfusion is diminished and the baby may become encephalopthic
- Reduced gut perfusion. The splanchnic blood vessels may vasoconstrict to maintain central BP. This relative gut ischaemia can predispose to NEC
Management of Hypotension
In general the mean arterial blood pressure (MABP) or the systolic BP is used to guide therapy. An open arterial duct (PDA) may cause a very low diastolic BP. A common rule of thumb is to maintain the minimum MABP above the infant’s gestational age in weeks + postnatal age in days, up to 4 days of age (e.g. a 27 week infant on day 2 of life should have a MABP no lower than 29 mmHg).
The thresholds at which to consider treating hypotension by birthweight are shown in Fig 16.1.
- Zone A. Treatment mandatory because the BP is critically low.
- Zone B. Possible hypotension and potential uncertainty, as the patient may either cope without support or show signs of failing organ perfusion. If clinical signs are present, such as metabolic acidosis, decreasing peripheral perfusion, poor capillary return, poor colour and low peripheral temperature, then treatment should commence.
- Zone C. Satisfactory BP; treatment required only if there are obvious clinical signs of poor perfusion.
In addition to the BP measurement there are two important factors to consider:
- What is the cause of the hypotension? This must be identified and treated.
- What is the best therapeutic option in restoring adequate BP?
In considering the first-line management of hypotension it is important to think through the possible physiological mechanisms:
- Preload: is the vascular compartment adequately filled? Is there volume depletion (e.g. blood loss)?
- Afterload: is this reduced (e.g. septic shock); should vascular resistance be increased?
- Contractility: is the myocardium working efficiently? Profound acidosis will affect muscle function.
- Heart rate: is it fast enough to maintain adequate cardiac output?
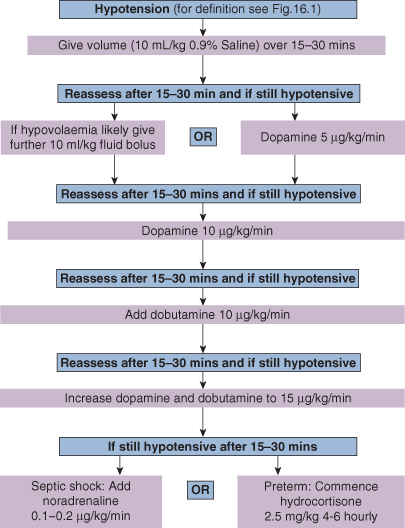
Figure 16.2 illustrates an approach to the incremental management of neonatal hypotension.
Volume Replacement
Many sick premature infants are hypovolaemic. It is difficult to measure circulating blood volume, but serum electrolytes, urinary specific gravity (SG), capillary refill time and daily weight may be helpful in assessment of circulating volume. If the baby is thought to be hypotensive due to volume depletion, an infusion of either a crystalloid or colloid should be given (10 mL/kg, repeated once if necessary). There is considerable controversy as to what fluid should be used. Normal saline (0.9%) is the first-line volume expander. If the vasculature is ‘leaky’ both crystalloid and colloid will be rapidly lost into the tissues, causing a further osmotic loss of fluid from the vascular compartment.
Giving fluid to a baby who already has increased preload may further decompensate cardiac output. Repeated fluid boluses should not be given in the absence of hypovolaemia. Measuring central venous pressure (CVP) via a central line or assessing cardiac filling by echocardiogram can be helpful.
Inotropic Agents
The action of dopamine depends on the dose. In low dose (1–5 µg kg−1 min−1) it primarily has dopaminergic actions and vasodilates the renal, coronary and possibly the cerebral circulation. In higher dose (5–10 µg kg−1 min−1) it stimulates β1 receptors, enhances myocardial contractility and increases heart rate. At yet higher dosage (10–20 µg kg−1 min−1) the main effects are α-adrenergic, with an increase in peripheral vascular resistance and a reduction in renal blood flow (see Table 16.1)
Dobutamine (5–20 µg kg−1 min−1 by continuous infusion) has mainly β2 effects, increasing BP by increasing myocardial contractility with some reduction in systemic resistance, thereby reducing afterload, which may be valuable in the failing heart. There is little effect on heart rate.
Noradrenaline (norepinephrine; 0.1–0.2 µg kg−1 min−1 by continuous infusion) is sometimes used once dopamine and dobutamine have been given in maximum dosage. It has both α and β effects, causing an increase in contractility, tachycardia and vascular resistance. Peripheral perfusion may become compromised.
Hydrocortisone (2.5 mg/kg 4–6 hourly) can be used to treat intractable hypotension in extreme preterm infants, some of whom have adrenal dysfunction. It has a slow onset of action and is associated with small bowel perforation, but can be life-saving.
Hypertension
Neonatal hypertension is not uncommon in sick neonates. It is rarely due to essential hypertension and can be associated with congenital malformations such as coarctation of the aorta, endocrine disorders, renal artery thrombosis, bronchopulmonary dysplasia (BPD) or steroid therapy. Table 16.2 lists the commoner causes of neonatal hypertension.
Table 16.2 Causes of neonatal hypertension
| Vascular | Renal artery thrombosis |
| Coarctation of the aorta | |
| Renal | Renal vein thrombosis |
| Renal dysplasia | |
| Obstructive uropathy | |
| Polycystic/multicystic disease | |
| Drugs | Corticosteroids |
| Methylxanthines | |
| Endocrine | Congenital adrenal hyperplasia (rare forms) |
| Phaeochromocytoma | |
| Neuroblastoma | |
| Miscellaneous | Bronchopulmonary dysplasia |
| Intracranial hypertension | |
| Convulsions | |
| Extracorporeal membrane oxygenation (ECMO) | |
| Essential |
Management is directed at the underlying cause, and may sometimes include renal or aortic surgery. Thrombolysis for renal artery thrombosis remains controversial; the potential benefit (rescuing the kidney) has to be balanced against the risk of intracranial haemorrhage.
The threshold at which anti-hypertensives should be commenced is difficult to define. Empirically systolic BP >90 mmHg (diastolic >60 mmHg) in a term infant and systolic >80 mmHg (diastolic >50 mmHg) in a preterm infant should be treated. The BP should be reduced slowly. Antihypertensives used include angiotensin-converting enzyme (ACE) inhibitors (e.g. captopril), nifedipine, beta-blockers (e.g. propranolol) and hydralazine.
Congenital Heart Disease
Congenital heart disease (CHD) refers to abnormal structure or function of the heart from birth. Many abnormalities remain asymptomatic and undetected in the neonatal period, only to be diagnosed weeks or years later. The generally accepted incidence is approximately 8/1000 live births, and CHD is the commonest form of major congenital abnormality in developed countries. Table 16.3 lists the frequency of different congenital cardiac anomalies. With advances in second trimester ultrasound examination, 70% of major congenital heart lesions are diagnosed antenatally.
Table 16.3 Contribution of the commoner congenital heart malformations to neonatal congenital heart disease
| Malformation | % of total |
| Ventricular septal defect | 25 |
| Patent ductus arteriosus | 15 |
| Atrial septal defect | 15 |
| Pulmonary stenosis | 10 |
| Aortic stenosis | 5 |
| Coarctation of the aorta | 5 |
| Transposition of the great arteries | 5 |
| Tetralogy of Fallot | 5 |
| Tricuspid atresia | 1 |
| Other individually rare conditions | 14 |
Aetiology
The aetiology of CHD is multifactorial and depends on the type of abnormality, but overall 75% have no identifiable cause. The main aetiological groups are shown in Table 16.4.
Table 16.4 Aetiological factors in congenital heart disease
| Factor | Examples |
| Chromosomal disorders (5% of all children with CHD) | Trisomy 21: 30–40% have CHD – AVSD, VSD, ToF, ASD |
| Trisomy 18: 90% have major cardiac defect, especially VSD | |
| Trisomy 13: 80% have major CHD – VSD | |
| Turner’s syndrome: 10% have coarctation of aorta, also aortic or mitral stenosis | |
| Single-gene defects (3% of CHD) | Noonan’s syndrome: pulmonary valve stenosis |
| Marfan’s syndrome: aortic valve disease and aortic dissection | |
| Holt–Oram syndrome: VSD and ASD | |
| Williams’ syndrome: supravalvular aortic stenosis | |
| DiGeorge syndrome (deletion 22q11): interrupted aortic arch, ToF | |
| Cornelia de Lange syndrome: VSD | |
| Associations with other defects | Oesophageal atresia: VACTERL |
| Pierre Robin: VSD | |
| CHARGE: ToF, AVSD, DORV, TGA | |
| Polygenic | Sibling with CHD = 2–5% risk, parent with CHD = 5–10% risk |
| Infection | Maternal rubella infection: coarctation, VSD, PDA |
| Drugs and teratogens | Maternal lithium is associated with Ebstein’s anomaly. Amphetamines, antimetabolites and anticonvulsants have been associated with CHD |
| Alcohol: 30% of infants with fetal alcohol syndrome have CHD | |
| Maternal conditions | Diabetes mellitus: VSD, TGA, hypertrophic cardiomyopathy |
| SLE: congenital heart block |
ASD, atrial septal defect; AVSD, atrioventricular septal defect; DORV, double outlet right ventricle; PDA, patent ductus arterioisus; ToF, tetralogy of Fallot; TGA, transposition of the great arteries; VSD, ventricular septal defect.
Mode of Presentation
Between 25 and 60% of CHD is detected on the standard ‘four-chamber’ view during the 20 week anomaly scan. With specialist fetal echocardiography the detection rate can rise to 85–90%. Fetal echocardiography is not yet widely available but is recommended for those with an antenatally detected anomaly or abnormal function such as hydrops or arrhythmia and for those with an increased nuchal translucency in the first trimester. After birth CHD is either detected by a murmur found on routine examination or by the baby developing symptoms of cyanosis or heart failure.
- Antenatally diagnosed: expected complex CHD should be delivered in a cardiac centre
- Murmur heard on routine examination in hospital or by family doctor
- Circulatory maladaptation at birth (especially PPHN)
- Cyanosis: respiratory causes of cyanosis must be quickly excluded
- Congestive cardiac failure: usually left-to-right shunts (e.g. VSD, PDA) or obstructive lesions (e.g. CoA).
- Dysrhythmias
- Low (postductal) oxygen saturation.
Heart Murmur Found on Newborn Examination
When a murmur is heard on routine examination in a healthy, asymptomatic infant, and no pathological features are present, then outpatient follow-up can be arranged. The parents will need reassurance. Most murmurs detected in the neonatal period will disappear in infancy, but if the murmur persists more than a few weeks or there are other abnormal signs then referral for echocardiography must be arranged.
Increasingly, the newborn examination includes a postductal saturation measurement, to detect duct-dependent lesions (see Chapter 6). Obstructive lesions will often present acutely as the duct closes within the first days of life. Heart failure due to left-to-right shunting presents later, at 3–6 weeks of age, as the pulmonary resistance falls. Dysrhythmias are relatively rare and may present antenatally (fetal tachycardia) or much later in childhood.
Investigations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree