- Respiratory distress
- Transient tachypnoea of the newborn
- Respiratory distress syndrome
- Pneumonia
- Pulmonary air leaks
- Meconium aspiration syndrome
- Pulmonary hypoplasia
- Pulmonary haemorrhage
- Congenital diaphragmatic hernia
- Oesophageal atresia and tracheo-oesophageal fistula
- Lobar emphysema
- Congenital cystic adenomatous malformation
- Chronic lung disease and bronchopulmonary dysplasia
Introduction
At birth, major cardiorespiratory changes take place in order to prepare the baby for extrauterine existence. This includes replacement of fetal lung fluid by air and establishment of regular spontaneous breathing. Associated with lung inflation and increased oxygenation there is a marked decrease in the pulmonary vascular resistance with consequent increased pulmonary blood flow and closure of the ductus arteriosus, foramen ovale and ductus venosus. As a result, the lungs take over the respiratory function previously carried out by the placenta. Many pathological processes can interfere with this normal sequence of events and cause respiratory disorders. Their clinical presentation varies according to the severity of the illness and depends on which part of the respiratory apparatus is primarily involved such as:
- respiratory distress (due to primary lung disease)
- apnoea and bradycardia (due to immaturity or dysfunction of respiratory centres in brain)
- stridor (due to upper airway obstruction)
- chronic respiratory insufficiency (due to fatigue or weakness of muscles of breathing such as in neuromuscular diseases, see Chapter 22).
Respiratory Distress
Respiratory distress is a general term used to describe respiratory symptoms and is not synonymous with respiratory distress syndrome (RDS). Signs of respiratory distress include:
- tachypnoea – a respiratory rate greater than 60/min
- expiratory grunt – breathing against a closed glottis
- chest retraction or recession
- flaring of the ala nasae
- cyanosis or low arterial oxygen saturation in air.
Diagnosis
The presence of two or more of the above signs persisting for 4 h or more suggests respiratory distress. There are many causes of respiratory distress in the newborn (Table 13.1).
Table 13.1 Common causes of respiratory distress in newborns
| Primary respiratory causes | Secondary to extrapulmonary pathology |
| TTN (or retained fetal lung fluid) | Following birth asphyxia: surgical conditions, e.g. choanal atresia, Pierre Robin sequence, diaphragmatic hernia, lobar emphysema, oesophageal atresia with TOF |
| RDS due to surfactant deficiency | Persistence of fetal circulation (or PPHN) |
| Pneumonia | Congenital heart disease |
| Pneumothorax; pneumomediastinum; pulmonary interstitial emphysema | Anaemia |
| Aspiration syndromes, e.g. meconium, milk, blood | Polycythaemia |
| Pulmonary hypoplasia, e.g. associated with oligohydramnios | Infection |
| Pulmonary haemorrhage | Metabolic disease |
| Chronic neonatal lung disease, e.g. BPD |
BPD, bronchopulmonary dysplasia; PPHN, persistent pulmonary hypertension of newborn; RDS, respiratory distress syndrome; TOF, tracheo-oesophageal fistula; TTN, transient tachypnoea of the newborn.
Diagnosis will be made by a full clinical history, physical examination and appropriate investigation, including a chest radiograph (Box 13.1). Perinatal history should include gestational age, the presence of polyhydramnios or oligohydramnios, anomalies on ultrasound, risk factors for sepsis, the passage of meconium, poor condition at birth and duration of amniotic membrane rupture. Physical examination includes observation of vital signs and auscultation of the lungs for symmetry of air entry, and heart sounds.
- Chest radiograph
- Bacteriological cultures on blood, urine and cerebrospinal fluid (CSF)
- Viral cultures and rapid-yield immunodiagnostic tests
- Haematocrit and full blood count
- Chest transillumination if pneumothorax suspected
- Passage of nasogastric catheters if choanal and oesophageal atresias suspected
- Hyperoxia test to differentiate between cardiac and respiratory disease
- Echocardiography
Treatment of Respiratory Distress
Supportive Care
The supportive care of the infant with respiratory distress is similar regardless of aetiology. Infants with respiratory distress require frequent or continuous observations of respiratory and heart rates, temperature, blood pressure and signs of respiratory distress. Accurate fluid balance charts are essential. Adequate temperature control and provision of nutrition essential part of respiratory care in newborns.
Oxygen
Oxygen is a useful and life-saving therapeutic agent, but is also potentially dangerous, particularly in the preterm baby, as it may damage the eyes (retinopathy of prematurity) and the lungs (bronchopulmonary dysplasia). When administered, it should be warmed to 34–37 °C and humidified. Oxygen therapy is discussed in Chapter 15.
Fluids
Infants with acute moderate to severe respiratory distress should not be enterally fed. With mild respiratory distress nasogastric feeding may be adequate, but with severe respiratory distress, intravenous fluids will be required. Usually a 10% dextrose saline solution or total parenteral nutrition (TPN) is used.
Monitoring of Blood Gases and Acid–Base Status
With moderate or severe respiratory distress, assessment of the arterial acid–base status, with samples from an intra-arterial catheter or capillary blood gases may be necessary. Continuous transcutaneous monitoring of PO2 and PCO2 decreases the requirement for blood sampling and enables rapid detection of fluctuations in clinical status. If respiratory acidosis is severe (pH <7.20 with PCO2 >60 mmHg, 8 kPa), assisted ventilation may be necessary. For a severe metabolic acidosis (base deficit more than −8), an infusion of sodium bicarbonate may be indicated but one should always try to treat the underlying cause first. Arterial catheterization is indicated when there is a need for frequent sampling for gas analysis, and is used for direct aortic blood pressure monitoring.
Artificial Respiratory Support
In more severe cases artificial respiratory support may be necessary. This may be in the form of non-invasive respiratory support such as continuous positive airway pressure (CPAP) which can be escalated to bilevel positive airway pressure (BiPAP) or non-invasive positive-pressure ventilation (NIPPV) if needed. Yet, in a proportion of cases, such treatment may fail and hence the necessity to switch to mechanical ventilation. This is described in Chapter 15.
Transient Tachypnoea of the Newborn
Transient tachypnoea of the newborn (TTN) occurs in approximately 1–2% of all newborn infants and is due to respiratory maladaptation at birth causing retention of fluids in the lungs (which are normally replaced by air by this time). Tachypnoea is generally the outstanding feature. TTN is usually benign and self-limiting, with symptoms rarely persisting beyond 48 h.
Pathogenesis
Most of the fetal lung fluid is squeezed out during descent through the birth canal or within the first few breaths after birth, but some is reabsorbed into the pulmonary capillaries and lymphatics. Occasionally, there is an excess of fluid or the clearance mechanisms are inefficient. In these cases retained fluid causes respiratory distress by making the lungs stiff.
Predisposing factors for TTN are late preterm delivery (34–36 weeks), heavy maternal analgesia, birth asphyxia, prelabour caesarean section, breech presentation, hypoproteinaemia and excessive fluid administration to the mother in labour. Polycythaemia may produce a similar clinical picture resulting from hyperviscosity with resultant pulmonary plethora. Maternal asthma is said to be associated with TTN.
Diagnosis
The diagnosis of TTN is confirmed by chest radiograph and the clinical course. Resolution of the respiratory distress within 48 h confirms the clinical diagnosis retrospectively. Chest radiograph shows streakiness caused by interstitial fluid, fluid in the lung fissures and perihilar cuffing. Pleural effusions and cardiomegaly may also sometimes be seen.
Treatment
TTN does not usually require respiratory support, other than extra inspired oxygen. Regular blood-gas measurements should be performed in the early stages of the illness. In more severe cases CPAP may aid resolution. If the blood gases deteriorate, the diagnosis should be reconsidered or complications such as pulmonary hypertension or pneumothorax may have developed. Sometimes the typical picture of TTN evolves over several days into one of RDS.
Respiratory Distress Syndrome
RDS is a specific clinical entity occurring predominantly but not exclusively in preterm infants owing to a lack of surfactant (a surface tension-lowering agent) in the alveoli. It has a characteristic clinical course and specific radiographic changes.
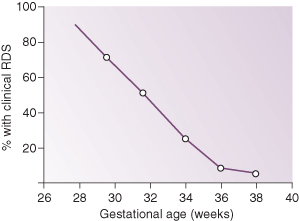
Incidence
Incidence of RDS is related to the degree of prematurity and is unusual in full-term infants (Fig. 13.1). Some predisposing factors are listed in Box 13.2.
- Prematurity
- Infant of a diabetic mother
- Antepartum haemorrhage
- Second twin
- Hypoxia, acidosis, shock
- Male sex
- Prelabour caesarean section (many factors involved)
Aetiology and Pathogenesis
The major hallmark of RDS is a deficiency of surfactant which leads to higher surface tension at the alveolar surface and interferes with the normal exchange of respiratory gases. The higher surface tension requires greater distending pressure to inflate the alveoli, according to the Laplace law:
(13.1) 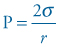
where P is the pressure required to inflate a sphere, σ the surface tension and r the radius.
Figure 13.2 Schematic representations of two alveoli demonstrating the Laplace law (see text for details).
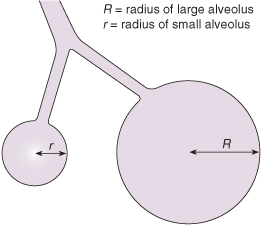
As the radius of the alveolus decreases (atelectasis) and as surface tension increases, the amount of pressure required to overcome these forces increases. The Laplace equation explains why, in the presence of high surface tension, large alveoli tend to become larger and small ones remain collapsed. Assuming that both small and large alveoli receive equal perfusion with blood, there will be a ventilation/perfusion (V/Q) imbalance. This results in severe biochemical disturbances, with hypoxia and acidosis, which give rise to a deterioration in pulmonary perfusion, thus causing further deterioration in V/Q. This may become progressively more severe and lead to persistent pulmonary hypertension (see Chapter 16).
Clinical Features
The signs of RDS start immediately after birth or become obvious in the first 6 h of life. In the absence of surfactant each breath the infant takes is like the first breath in an effort to expand the alveoli. Fatigue contributes to the respiratory failure. The clinical course is usually associated with worsening of the symptoms, with a peak severity at 48–72 h, although occasionally maximum severity may occur in infants less than 12 h old. As the disease progresses the infant shows a need for increasing oxygen, the expiratory grunt may diminish and prolonged apnoea may occur. Without intervention, recurrent and worsening apnoea superimposed on tachypnoea would indicate impending respiratory failure and the need for mechanical ventilation. The breath sounds are decreased and there may be a fall in blood pressure. The infant is oliguric initially and has evidence of increasing peripheral oedema due to fluid retention. At about 48 h a diuresis often occurs, with a concomitant clinical improvement in less severely affected infants.
It should, however, be realized that presentation of RDS in babies born at extreme preterm gestations, such as those born at 23 and 24 weeks’ gestation, may have an entirely different pattern of clinical presentation and course of illness. Such babies may not show signs of respiratory distress. Their clinical condition appears quite stable with a normal chest radiograph. This, however, lasts for a short period of 2–3 days when their respiratory problem starts and they run into problems with prolonged ventilatory dependency and chronic lung disease. This may be because such infants are born with lungs in very early stages of development (see Chapter 15) which are more susceptible to lung injury particularly if requiring ventilation and oxygen treatment (pulmonary injury sequence).
Radiology
The radiograph is characteristic, showing underaeration and a fine reticular or granuloreticular pattern, often referred to as ‘ground glass’. In addition, air bronchograms are seen in the lung fields (Fig. 13.3). Severe cases with near-total atelectasis may show complete opacification of the lung fields (‘white out’). The appearance of the chest radiograph may not correlate well with the clinical severity of RDS. Extremely preterm infants, in whom lungs are not fully developed, may actually have normal lung fields.
Laboratory Abnormalities
Arterial oxygen tension is usually decreased. Arterial carbon dioxide tension may initially be normal (when breathing faster to compensate for respiratory difficulty), but is usually increased. Blood pH may reflect respiratory acidosis (from hypercarbia), metabolic acidosis (from tissue hypoxia) or mixed acidosis.
Treatment
Surfactant Replacement Therapy
The development and use of surfactant replacement therapy has revolutionized the treatment of RDS. Numerous preparations, including animal-derived surfactants and equally effective newer synthetic surfactants, have been developed and tested in numerous randomized controlled trials, all of which confirm the efficacy of surfactant in improving survival and reducing complications such as pneumothorax.
Surfactants can be used either ‘prophylactically’ in at risk infants (such as those born less than 27 weeks’ gestation) or as a ‘rescue’ treatment whenever babies develop respiratory distress. Different surfactant preparations have different composition and dosing schedule but they are all effective. Normally one or two doses of surfactant should be sufficient and there is no evidence that further doses provide any real benefit.
Establishment of Adequate Gas Exchange
Oxygen
The aim of treatment is to maintain the PaO2 within the normal range. This is done in very mild cases by increasing the inspired oxygen concentration (FIO2). In more severe cases respiratory support may be necessary, but the further management depends on the size of the infant and degree of gaseous exchange abnormality.
Although CPAP may be administered shortly after birth, smaller infants are more likely to require mechanical ventilation. Indications for mechanical ventilation are listed in Box 13.3.
- Apnoea of increasing severity;
- FIO2 exceeding 60% to maintain PaO2 >60 mmHg (8 kPa);
- Rising PaCO2 that exceeds 60 mmHg (8 kPa), particularly if there is a falling pH (<7.20).
Supportive Treatment
Chest Physiotherapy
Infants receive regular position changes and airway suctioning to reduce the risk of mucus retention, airway plugging and pulmonary collapse. Routine chest physiotherapy is of no proven value in management of RDS.
Antibiotics
Broad-spectrum antibiotics are usually administered to all babies with RDS after suitable cultures have been taken. This is because sepsis/pneumonia, especially group B streptococcal infection, can produce a nearly identical picture. Antibiotics should be stopped once cultures, blood count and ancillary investigations for sepsis are reported as negative. The choice of antibiotics depends on the local preference keeping in mind the prevalence of local bacteriological spectrum.
Appropriate Fluid Balance
See Chapter 9.
Blood Pressure Monitoring
See Chapter 16.
Minimal Handling and Nursing in a Neutral Environmental Temperature
See Chapter 24.
Complications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree