Technology and Safety
The real-time image on the ultrasound screen is produced by sound waves that are reflected back from fluid and tissue interfaces of the fetus, amnionic fluid, and placenta. Sector array transducers used in obstetrics contain groups of piezoelectric crystals working simultaneously in arrays. These crystals convert electrical energy into sound waves, which are emitted in synchronized pulses. Sound waves pass through tissue layers and are reflected back to the transducer when they encounter an interface between tissues of different densities. Dense tissue such as bone produces high-velocity reflected waves, which are displayed as bright echoes on the screen. Conversely, fluid generates few reflected waves and appears dark—or anechoic. Digital images generated at 50 to more than 100 frames per second undergo postprocessing that yields the appearance of real-time imaging.
Ultrasound refers to sound waves traveling at a frequency above 20,000 hertz (cycles per second). Higher-frequency transducers yield better image resolution, whereas lower frequencies penetrate tissue more effectively. Transducers use wide-bandwidth technology to perform over a range of frequencies. In the second trimester, a 4- to 6-megahertz abdominal transducer is often in close enough proximity to the fetus to provide precise images. By the third trimester, however, a lower frequency 2- to 5-megahertz transducer may be needed for penetration, but can lead to compromised resolution. This explains why resolution is often poor when imaging obese patients and why low-frequency transducers are needed to reach the fetus through maternal tissues. In early pregnancy, a 5- to 10-megahertz vaginal transducer may provide excellent resolution, because the early fetus is close to the transducer.
Fetal Safety
Sonography should be performed only for a valid medical indication, using the lowest possible exposure setting to gain necessary information—the ALARA principle—as low as reasonably achievable. It should be performed only by those trained to recognize medically important conditions such as fetal anomalies, artifacts that may mimic pathology, and techniques to avoid ultrasound exposure beyond what is considered safe for the fetus (American Institute of Ultrasound in Medicine, 2008a, 2013a). Prolonged ultrasound exposure may affect brain cell migration in fetal mice (Rakic, 2006). However, no causal relationship has been demonstrated between diagnostic ultrasound and recognized adverse effects in human pregnancy (American Institute of Ultrasound in Medicine, 2010).
All sonography machines are required to display two indices: the thermal index and the mechanical index. The thermal index is a measure of the relative probability that the examination may raise the temperature, potentially enough to induce injury. That said, it is extremely unlikely that fetal damage could occur using commercially available ultrasound equipment in routine practice. The potential for temperature elevation is higher with longer examination time and is greater near bone than in soft tissue. Also, theoretical risks are greater during organogenesis than later in gestation. The thermal index is higher with pulsed Doppler applications than with routine B-mode scanning (p. 219). In the first trimester, if pulsed Doppler is needed for a clinical indication, the thermal index should be ≤ 1.0, and exposure time should be kept as short as possible, usually no longer than 5 to 10 minutes (American Institute of Ultrasound in Medicine, 2011; Salvesen, 2011). To document the embryonic or fetal heart rate, M-mode imaging should be used instead of pulsed Doppler imaging (American Institute of Ultrasound in Medicine, 2013a).
The mechanical index is a measure of likelihood of adverse effects related to rarefractional pressure, such as cavitation—which is relevant only in tissues that contain air. Microbubble ultrasound contrast agents are not used in pregnancy for this reason. No adverse effects have been reported in mammalian tissues that do not contain gas bodies over the range of diagnostically relevant exposures. Because fetuses cannot contain gas bodies, they are not considered at risk (American Institute of Ultrasound in Medicine, 2008b).
The use of sonography for any nonmedical purpose, such as “keepsake fetal imaging,” is considered contrary to responsible medical practice and is not condoned by the Food and Drug Administration (Rados, 2007), the American Institute of Ultrasound in Medicine (2012, 2013a), or the International Society of Ultrasound in Obstetrics and Gynecology (2011).
Operator Safety
The reported prevalence of work-related musculoskeletal discomfort or injury among sonographers and sonologists is as high as 70 to 80 percent (Janga, 2012; Magnavita, 1999; Pike, 1997). According to the National Institute for Occupational Safety and Health, the main risk factors for injury during transabdominal ultrasound are awkward posture, sustained static forces, and various pinch grips while maneuvering the transducer (Centers for Disease Control and Prevention, 2006). Another possible contributory factor is maternal habitus—as more force may be employed when imaging obese patients.
The following guidelines may help avert injury:
1. Position the patient on the examination table close to you, so that your elbow is close to your body, with less than 30 degrees shoulder abduction, keeping your thumb facing up.
2. Adjust the table or chair height so that your forearm is parallel to the floor.
3. If seated, use a chair with back support, support your feet, and keep ankles in neutral position. Do not lean toward the patient or monitor.
4. Face the monitor squarely and position it so that it is viewed at a neutral angle, such as 15 degrees downward.
5. Avoid reaching, bending, or twisting while scanning.
6. Frequent breaks may avoid muscle strain. Stretching and strengthening exercises can be helpful.
 First-Trimester Sonography
First-Trimester Sonography
Indications for sonography before 14 weeks’ gestation are listed in Table 10-1. Early pregnancy can be evaluated using transabdominal or transvaginal sonography, or both. The components listed in Table 10-2 should be assessed. The crown-rump length (CRL) is the most accurate biometric predictor of gestational age (Appendix, p. 1294). The CRL should be obtained in a sagittal plane and include neither the yolk sac nor a limb bud. If carefully performed, it has a variance of only 3 to 5 days.
TABLE 10-1. Some Indications for First-Trimester Ultrasound Examination
Confirm an intrauterine pregnancy
Evaluate a suspected ectopic pregnancy
Define the cause of vaginal bleeding
Evaluate pelvic pain
Estimate gestational age
Diagnose or evaluate multifetal gestations
Confirm cardiac activity
Assist chorionic villus sampling, embryo transfer, and localization and removal of an intrauterine device
Assess for certain fetal anomalies such as anencephaly, in high-risk patients
Evaluate maternal pelvic masses and/or uterine abnormalities
Measure nuchal translucency when part of a screening program for fetal aneuploidy
Evaluate suspected gestational trophoblastic disease
TABLE 10-2. Components of Standard Ultrasound Examination by Trimester
First-trimester sonography can reliably diagnose anembryonic gestation, embryonic demise, ectopic pregnancy, and gestational trophoblastic disease. Multifetal gestation can be identified early, and this is the optimal time to determine chorionicity (Chap. 45, p. 896). The first trimester is also the ideal time to evaluate the uterus, adnexa, and cul-de-sac. Cervical length and the relationship of the placenta to the cervical os are best evaluated in the second trimester.
An intrauterine gestational sac is reliably visualized with transvaginal sonography by 5 weeks, and an embryo with cardiac activity by 6 weeks. The embryo should be visible transvaginally once the mean sac diameter has reached 20 mm—otherwise the gestation is anembryonic (Chap. 18, p. 355). Cardiac motion is usually visible with transvaginal imaging when the embryo length has reached 5 mm. If an embryo less than 7 mm is not identified to have cardiac activity, a subsequent examination is recommended in 1 week (American Institute of Ultrasound in Medicine, 2013a). At Parkland hospital, first-trimester demise is diagnosed if the embryo has reached 10 mm without cardiac motion, taking into consideration the standard error of the ultrasound measurements.
Nuchal Translucency (NT)
Nuchal translucency evaluation, a component of first-trimester aneuploidy screening, has had a major impact on the number of pregnancies receiving late first-trimester ultrasound examination. It represents the maximum thickness of the subcutaneous translucent area between the skin and soft tissue overlying the fetal spine at the back of the neck (Fig. 14-5, p. 290). It is measured in the sagittal plane between 11 and 14 weeks using precise criteria (Table 10-3). When the nuchal translucency is increased, the risk for fetal aneuploidy and various structural anomalies—including heart defects—is significantly elevated. Aneuploidy screening using nuchal translucency measurement in conjunction with assessment of maternal serum human chorionic gonadotropin and pregnancy-associated plasma protein A levels is discussed in Chapter 14 (p. 290).
TABLE 10-3. Guidelines for Nuchal Translucency (NT) Measurement
The margins of NT edges must be clear enough for proper caliper placement
The fetus must be in the midsagittal plane
The image must be magnified so that it is filled by the fetal head, neck, and upper thorax
The fetal neck must be in a neutral position, not flexed and not hyperextended
The amnion must be seen as separate from the NT line
Electronic calipers must be used to perform the measurement
The + calipers must be placed on the inner borders of the nuchal space with none of the horizontal crossbar itself protruding into the space
The calipers must be placed perpendicular to the long axis of the fetus
The measurement must be obtained at the widest space of the NT
First-Trimester Fetal Anomaly Detection
Assessment for selected fetal abnormalities in an at-risk pregnancy is another indication for first-trimester sonography (see Table 10-1). Research in this area has focused on anatomy visible at 11 to 14 weeks, to coincide with sonography performed as part of aneuploidy screening (Chap. 14, p. 289). With current technology, it is not realistic to expect that all major abnormalities detectable in the second trimester may be visualized in the first trimester. A study of systematic anatomy evaluation between 11 and 14 weeks in more than 40,000 pregnancies yielded a detection rate of approximately 40 percent for nonchromosomal abnormalities (Syngelaki, 2011). This detection rate is nearly identical to that from a review of more than 60,000 pregnancies from 15 studies and is also comparable with other reports (Pilalis, 2012; Syngelaki, 2011). Identification varies considerably according to the specific abnormality. For example, reported detection rates are extremely high for anencephaly, alobar holoprosencephaly, and ventral wall defects. However, only one-third of major cardiac anomalies have been identified, with no detected cases of microcephaly, agenesis of the corpus callosum, cerebellar abnormalities, congenital pulmonary airway malformations, or bowel obstruction (Syngelaki, 2011). Thus, as first-trimester sonography is unreliable for detection of many major abnormalities, it should not replace second-trimester anatomical evaluation.
 Second- and Third-Trimester Sonography
Second- and Third-Trimester Sonography
The many indications for second- and third-trimester sonography are listed in Table 10-4. There are three types of examinations: standard, specialized, and limited.
TABLE 10-4. Indications for Second- or Third-Trimester Ultrasound Examination
Maternal Indications
Vaginal bleeding
Abdominal/pelvic pain
Pelvic mass
Suspected uterine abnormality
Suspected ectopic pregnancy
Suspected molar pregnancy
Suspected placenta previa and subsequent surveillance
Suspected placental abruption
Preterm premature rupture of membranes and/or preterm labor
Cervical insufficiency
Adjunct to cervical cerclage
Adjunct to amniocentesis or other procedure
Adjunct to external cephalic version
Fetal Indications
Gestational age estimation
Fetal-growth evaluation
Significant uterine size/clinical date discrepancy
Suspected multifetal gestation
Fetal anatomical evaluation
Fetal anomaly screening
Assessment for findings that may increase the aneuploidy risk
Abnormal biochemical markers
Fetal presentation determination
Suspected hydramnios or oligohydramnios
Fetal well-being evaluation
Follow-up evaluation of a fetal anomaly
History of congenital anomaly in prior pregnancy
Suspected fetal death
Fetal condition evaluation in late registrants for prenatal care
1. Standard sonographic examination is the most commonly performed. Components are listed in Table 10-2. The fetal anatomical structures that should be evaluated during the examination, which are listed in Table 10-5, may be adequately assessed after approximately 18 weeks. When examining twins or other multiples, documentation also includes the number of chorions and amnions, comparison of fetal sizes, estimation of amnionic fluid volume within each sac, and fetal sex determination (Chap. 45, p. 912).
2. There are several types of specialized examinations. The targeted examination is a detailed anatomical survey performed when an abnormality is suspected on the basis of history, screening test result, or abnormal findings from a standard examination (American Institute of Ultrasound in Medicine, 2013a). A targeted examination is performed and interpreted by an experienced operator. It includes the anatomical structures listed in Table 10-5, along with additional views of the brain and cranium, neck, profile, lungs and diaphragm, cardiac anatomy, liver, shape and curvature of the spine, hands and feet, and any placental abnormalities. The physician performing the examination further determines whether other examination components will be needed on a case-by-case basis (American College of Obstetricians and Gynecologists, 2011). Other specialized examinations include fetal echocardiography and Doppler evaluation (discussed below), biophysical profile (Chap. 17, p. 341), and additional biometric measurements.
3. A limited examination is performed to address a specific clinical question. Examples include amnionic fluid volume assessment, placental location, or evaluation of fetal presentation or viability. In most cases, a limited examination is appropriate only when a prior standard or targeted examination has previously been performed (American Institute of Ultrasound in Medicine, 2009, 2013a).
TABLE 10-5. Minimal Elements of a Standard Examination of Fetal Anatomy
Head, face, and neck
Lateral cerebral ventricles
Choroid plexus
Midline falx
Cavum septum pellucidi
Cerebellum
Cisterna magna
Upper lip
Consideration of nuchal fold measurement at 15–20 weeks
Chest
Four-chamber view of the heart
Left ventricular outflow tract
Right ventricular outflow tract
Abdomen
Stomach—presence, size, and situs
Kidneys
Urinary bladder
Umbilical cord insertion into fetal abdomen
Umbilical cord vessel number
Spine
Cervical, thoracic, lumbar, and sacral spine
Extremities
Legs and arms
Fetal sex
In multifetal gestations and when medically indicated
Fetal Biometry
Equipment software derives the estimated gestational age from the crown-rump length. Formulas are similarly used to calculate estimated gestational age and fetal weight from measurements of the biparietal diameter, head and abdominal circumference, and femur length (Fig. 10-1). The estimates are most accurate when multiple parameters are used and when nomograms derived from fetuses of similar ethnic or racial background living at similar altitude are selected. Even the best models may over- or underestimate fetal weight by as much as 15 percent (American Institute of Ultrasound in Medicine, 2013a). Various nomograms for other fetal structures, including the cerebellum diameter, ear length, interocular and binocular distances, thoracic circumference, and kidney, long bones, and feet lengths, may be used to address specific questions regarding organ system abnormalities or syndromes. These nomograms may be found in the Appendix (p. 1298).
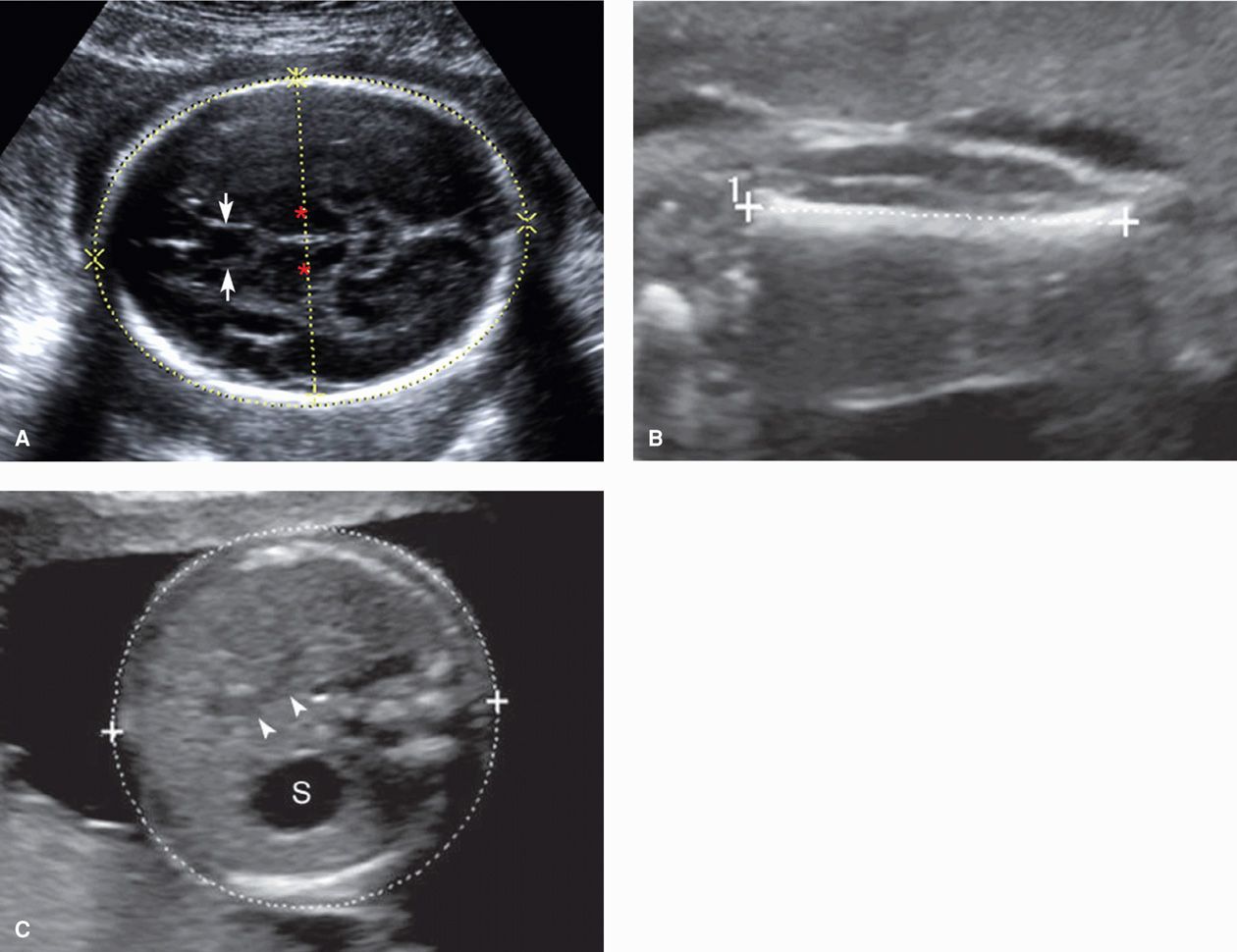
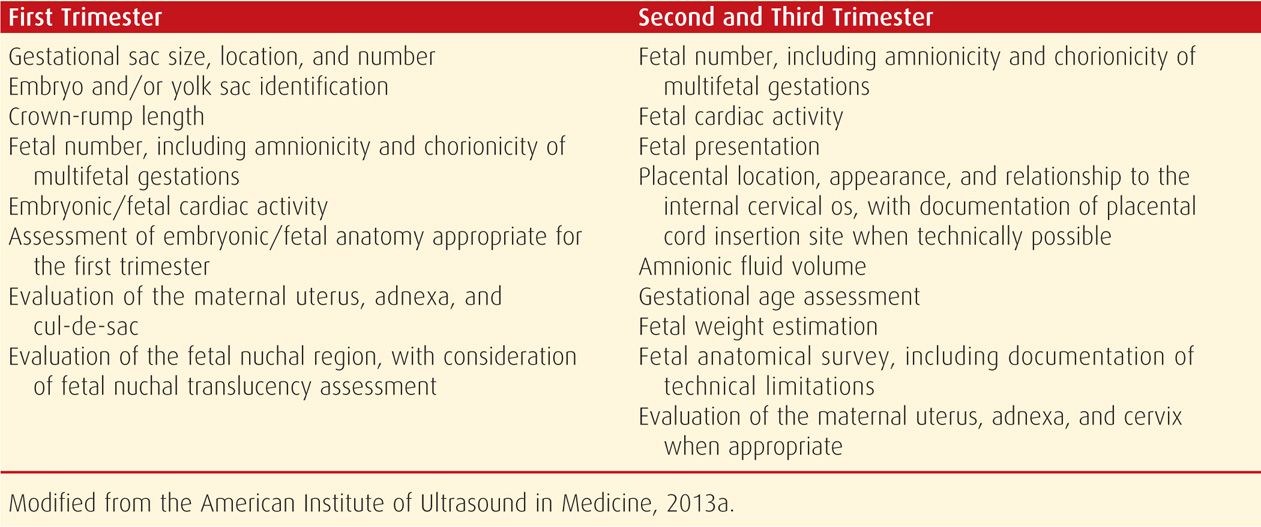
FIGURE 10-1 Fetal biometry. A. Transthalamic view. A transverse (axial) image of the head is obtained at the level of the cavum septum pellucidum (arrows) and thalami (asterisks). The biparietal diameter is measured perpendicular to the sagittal midline, from the outer edge of the skull in the near-field to the inner edge of the skull in the far-field. By convention, the near-field is that which is closer to the sonographic transducer. The head circumference is measured circumferentially around the outer border of the skull. B. Femur length. The femur is measured perpendicular to the femoral shaft, from each diaphyseal end, excluding the epiphysis. C. Abdominal circumference. This is a transverse measurement at the level of the stomach (S). The J-shaped structure (arrowheads) indicates the confluence of the umbilical vein and the right portal vein. Ideally, only one rib is visible on each side of the abdomen, indicating that the image was not taken at an oblique angle.
In the second trimester, the biparietal diameter (BPD) most accurately reflects the gestational age, with a variation of 7 to 10 days. The BPD is measured in the transthalamic view, at the level of the thalami and cavum septum pellucidum (CSP), from the outer edge of the skull in the near field to the inner edge of the skull in the far field (see Fig. 10-1A). The head circumference (HC) is also measured in the transthalamic view, either by placing an ellipse around the outer edge of the skull, or by measuring the occipital-frontal diameter (OFD) and calculating the circumference from the BPD and OFD. The cephalic index, which is the BPD divided by the OFD, is normally approximately 70 to 86 percent. If the head shape is flattened—dolichocephaly, or rounded—brachycephaly, the HC is more reliable than the BPD. Dolichocephaly and brachycephaly may be normal variants or may be secondary to positional changes or oligohydramnios. However, dolichocephaly can occur with neural-tube defects, and brachycephaly may be seen in fetuses with Down syndrome (Chap. 13, p. 262). Whenever the skull shape is abnormal, craniosynostosis and other craniofacial abnormalities are a consideration.
The femur length (FL) correlates well with both BPD and gestational age. It is measured with the beam perpendicular to the long axis of the shaft, excluding the epiphysis. For gestational age estimation, it has a variation of 7 to 11 days in the second trimester (see Fig. 10-1B). A mildly foreshortened femur—one that is 90 percent or less than that expected for gestational age—has been used as a minor marker for Down syndrome (Chap. 14, p. 292). A femur length that is dramatically foreshortened prompts an evaluation for a skeletal dysplasia, as discussed on page 217. In general, the normal range for the FL to abdominal circumference (AC) ratio is 20 to 24 percent. A FL/AC < 16 percent suggests a lethal skeletal dysplasia, particularly if other skeletal abnormalities are present (Rahemtullah, 1997; Ramus, 1998).
The abdominal circumference has the greatest variation, up to 2 to 3 weeks, for gestational age estimation. The AC is measured around the outer border of the skin. This is a transverse image at the level of the stomach and the confluence of the umbilical vein with the portal sinus (see Fig. 10-1C). Of the biometric parameters, AC is most affected by fetal growth. As discussed in Chapter 44 (p. 875), a small abdominal circumference has been used as an early indicator of fetal-growth restriction (Baschat, 2011).
Variability of the estimated gestational age and of fetal weight increases with advancing gestation. Individual measurements are least accurate in the third trimester. Although estimates are improved by averaging multiple parameters, if one parameter differs significantly from the others, consideration is given to excluding it from the calculation. The outlier could result from poor visibility, but it could also indicate a fetal abnormality or growth problem. Reference tables such as the one in the Appendix (p. 1296) are used to estimate fetal weight percentiles. Menstrual dates are generally considered confirmed if an estimated gestational age (EGA) based on a sonographic first-trimester crown-rump length is within 1 week or if the EGA from biometry at 14 to 20 weeks is within 10 days (American College of Obstetricians and Gynecologists, 2011). In the third trimester, the accuracy of sonography is only within 3 to 4 weeks. Sonographic evaluation performed to monitor fetal growth should typically be performed at least 2 to 4 weeks after a prior examination (American Institute of Ultrasound in Medicine, 2013a).
Amnionic Fluid
Amnionic fluid volume evaluation is a component of every second- or third-trimester sonogram. Oligohydramnios indicates that the volume is below normal range, and subjective crowding of the fetus is often noted. Hydramnios—also called polyhydramnios—is defined as amnionic fluid volume above normal (Fig. 11-3, p. 234). Although it is considered acceptable for an experienced examiner to assess the amnionic fluid volume qualitatively, fluid is usually assessed semiquantitatively (American Institute of Ultrasound in Medicine, 2013a). Measurements include either the single deepest vertical fluid pocket or the sum of the deepest vertical pockets from each of four equal uterine quadrants—the amnionic fluid index (Phelan, 1987). Reference ranges have been established for both measurements from 16 weeks’ gestation onward (Fig. 11-1, p. 233). The single deepest vertical pocket is normally between 2 and 8 cm, and the amnionic fluid index normally ranges between 8 and 24 cm. Amnionic fluid volume is discussed further in Chapter 11 (p. 232).
Fetal Anatomical Evaluation
An important goal of second- and third-trimester sonography is to systematically evaluate fetal anatomy and determine whether specific anatomical components appear normal or abnormal. If a single ultrasound examination is planned for the purpose of evaluating fetal anatomy, the American College of Obstetricians and Gynecologists (2011) recommends that it be performed at 18 to 20 weeks. At this gestational age range, complex organs such as the fetal brain and heart can be imaged clearly enough to visualize many major malformations. Technical factors such as maternal habitus, abdominal wall scarring, fetal size, and fetal position may limit adequate visualization, and these limitations should be noted in the report. If imaging is suboptimal, a follow-up examination may be helpful. If an abnormality is identified or suspected during a standard examination of fetal anatomy, specialized sonography is indicated.
Second-Trimester Fetal Anomaly Detection. The sensitivity of sonography for detecting fetal anomalies varies according to factors such as gestational age, maternal habitus, fetal position, equipment features, examination type, operator skill, and the specific abnormality in question. For example, maternal obesity has been associated with a 20-percent reduction in the fetal anomaly detection rate, regardless of examination type (Dashe, 2009).
Imaging technological advances have contributed to dramatic improvements in anomaly detection. In a review of more than 925,000 pregnancies evaluated between 1978 and 1997, Levi (2002) identified an overall anomaly detection rate of 40 percent. The single largest trial, the EUROFETUS study, included 170,800 pregnancies and identified 55 percent with severe malformations before 24 weeks (Grandjean, 1999). The most recent data are available from a network of population-based registries from 21 European countries, termed EUROCAT, found at: www.eurocat-network.eu.
Between 2006 and 2010, EUROCAT prenatal detection rates for selected anomalies were as follows: anencephaly, 97 percent; spina bifida, 84 percent; hydrocephaly, 77 percent; cleft lip, 54 percent; hypoplastic left heart, 73 percent; diaphragmatic hernia, 59 percent; gastroschisis, 94 percent; omphalocele, 84 percent; bilateral renal agenesis, 91 percent; posterior urethral valves, 81 percent; limb-reduction defects, 52 percent; and clubfoot, 43 percent (EUROCAT, 2012). Importantly, however, the overall anomaly detection rate, excluding aneuploidy, was only 34 percent. This reflects inclusion of anomalies with minimal or no sonographic detection, such as microcephaly, anotia, choanal atresia, cleft palate, bile duct atresia, Hirschsprung disease, anal atresia, and congenital skin disorders. These are mentioned because clinicians tend to focus on abnormalities amenable to sonographic detection, whereas families may find those not readily detectable no less devastating. Every sonographic examination should include a frank discussion of examination limitations.
The sensitivity of specialized sonography in experienced centers is considered to be at least 80 percent (American College of Obstetricians and Gynecologists, 2011). Most anomalous infants—approximately 75 percent—occur in pregnancies that are otherwise low-risk, that is, without an indication for specialized sonography. Thus, the quality of a standard screening sonogram greatly affects overall anomaly detection from a population perspective (Dashe, 2009; Levi, 2002). Practice guidelines and standards established by organizations such as the American Institute of Ultrasound in Medicine (2013a) and the International Society of Ultrasound in Obstetrics and Gynecology (Salomon, 2011) have undoubtedly contributed to improvements in anomaly detection rates.
NORMAL AND ABNORMAL FETAL ANATOMY
Many fetal anomalies and syndromes may be characterized with targeted sonography. Selected abnormalities of the anatomical components in Table 10-5 are discussed below. This list is not intended to be comprehensive but covers abnormalities that are relatively common and may be detectable with standard sonography, as well as those that are potentially amenable to fetal therapy. Sonographic features of chromosomal abnormalities are reviewed in Chapters 13 and 14, and fetal therapy is discussed in Chapter 16.
 Brain and Spine
Brain and Spine
Standard sonographic evaluation of the fetal brain includes three transverse (axial) views. The transthalamic view is used to measure the BPD and HC and includes the midline falx, cavum septum pellucidum (CSP), and thalami (see Fig. 10-1A). The CSP is the space between the two laminae that separate the frontal horns. Inability to visualize a normal CSP may indicate a midline brain abnormality such as agenesis of the corpus callosum, lobar holoprosencephaly, or septo-optic dysplasia (de Morsier syndrome). The transventricular view includes the lateral ventricles, which contain the echogenic choroid plexus (Fig. 10-2). The ventricles are measured at their atrium, which is the confluence of their temporal and occipital horns. The transcerebellar view is obtained by angling the transducer back through the posterior fossa (Fig. 10-3). In this view, the cerebellum and cisterna magna are measured, and between 15 and about 20 weeks, the nuchal skinfold thickness may be measured (Chap. 14, p. 289). From 15 until 22 weeks, the cerebellar diameter in millimeters is roughly equivalent to the gestational age in weeks (Goldstein, 1987). The cisterna magna normally measures between 2 and 10 mm. Effacement of the cisterna magna is present in the Chiari II malformation, discussed on page 202.
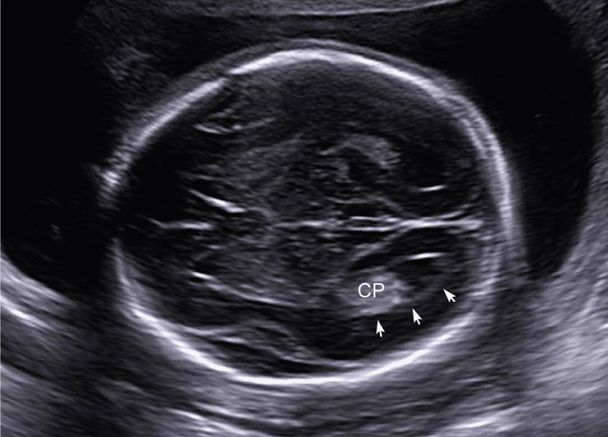
FIGURE 10-2 The transventricular view depicts the lateral ventricles, which contain the echogenic choroid plexus (CP). The lateral ventricle is measured at the atrium (arrows), which is the confluence of the temporal and occipital horns. A normal measurement is between 5 and 10 mm throughout the second and third trimesters. The atria measured 6 mm in this 21-week fetus.
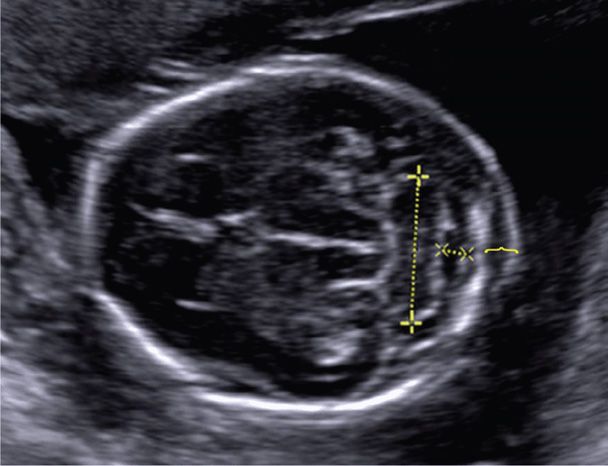
FIGURE 10-3 Transcerebellar view of the posterior fossa, demonstrating measurement of the cerebellum (+), cisterna magna (X), and nuchal fold thickness (bracket). Care is taken not to angle obliquely down the spine, which may artificially increase the nuchal fold measurement.
Imaging of the spine includes evaluation of the cervical, thoracic, lumbar, and sacral regions (Fig. 10-4). Representative spinal images for record keeping are often obtained in the sagittal or coronal plane. However, real-time imaging should include evaluation of each spinal segment in the transverse plane, as this is more sensitive for abnormality detection. Transverse images demonstrate three ossification centers. The anterior ossification center is the vertebral body, and the posterior paired ossification centers represent the junction of vertebral laminae and pedicles. Ossification of the spine proceeds in a cranial-caudal fashion, such that ossification of the upper sacrum (S1-S2) is not generally visible sonographically before 16 weeks’ gestation. Ossification of the entire sacrum may not be visible until 21 weeks (De Biasio, 2003). Thus, detection of some spinal abnormalities may be challenging in the early second trimester.
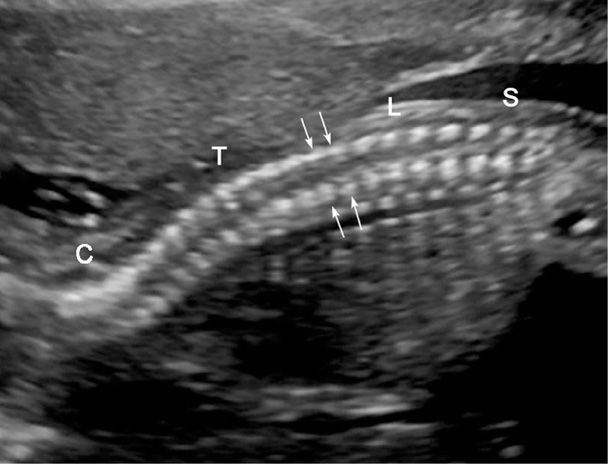
FIGURE 10-4 Normal fetal spine. In this sagittal image of a 21-week fetus, the cervical (C), thoracic (T), lumbar (L), and sacral spine (S) are depicted. Arrows denote the parallel rows of paired posterior ossification centers—representing the junction of vertebral lamina and pedicles.
Examples of spinal abnormalities include spina bifida, caudal regression sequence, and sacrococcygeal teratoma. More subtle spinal abnormalities may be visible. These include diastematomyelia, which is a longitudinal cleft or splitting of the spinal cord itself, and hemivertebrae—a component of the vertebral, anal, cardiac, tracheo-esophageal fistula, renal, limb (VACTERL) association.
If a brain or spinal abnormality is identified, specialized sonography is indicated. The International Society of Ultrasound in Obstetrics and Gynecology (2007) has published guidelines for a “fetal neurosonogram.” Fetal magnetic resonance (MR) imaging may be helpful in further characterizing central nervous system (CNS) abnormalities (p. 223).
Neural-Tube Defects
These result from incomplete closure of the neural tube by the embryonic age of 26 to 28 days. They are the second most common class of malformations after cardiac anomalies. Their prevalence was previously considered to be 1.4 to 2 per 1000 births. However, birth defect registries in the United States and Europe now report a prevalence of only 0.9 per 1000. In the United Kingdom, the prevalence is 1.3 per 1000 (Dolk, 2010). Neural-tube defects can be prevented with folic acid supplementation (Chap. 9, p. 181), and their prenatal diagnosis is discussed in Chapter 14 (p. 283). When isolated, neural-tube defect inheritance is multifactorial, and the defect recurrence risk without periconceptional folic acid supplementation is 3 to 5 percent.
Anencephaly is characterized by absence of the cranium and telencephalic structures, with the skullbase and orbits covered only by angiomatous stroma. Acrania is absence of the cranium, with protrusion of disorganized brain tissue. Both are generally grouped together, and anencephaly is considered to be the final stage of acrania (Bronshtein, 1991; McGahan, 2003). These lethal anomalies can be diagnosed in the late first trimester, and with adequate visualization, virtually all cases may be diagnosed in the second trimester (Fig. 10-5). An inability to view the biparietal diameter should raise suspicion. Hydramnios from impaired fetal swallowing is common in the third trimester.
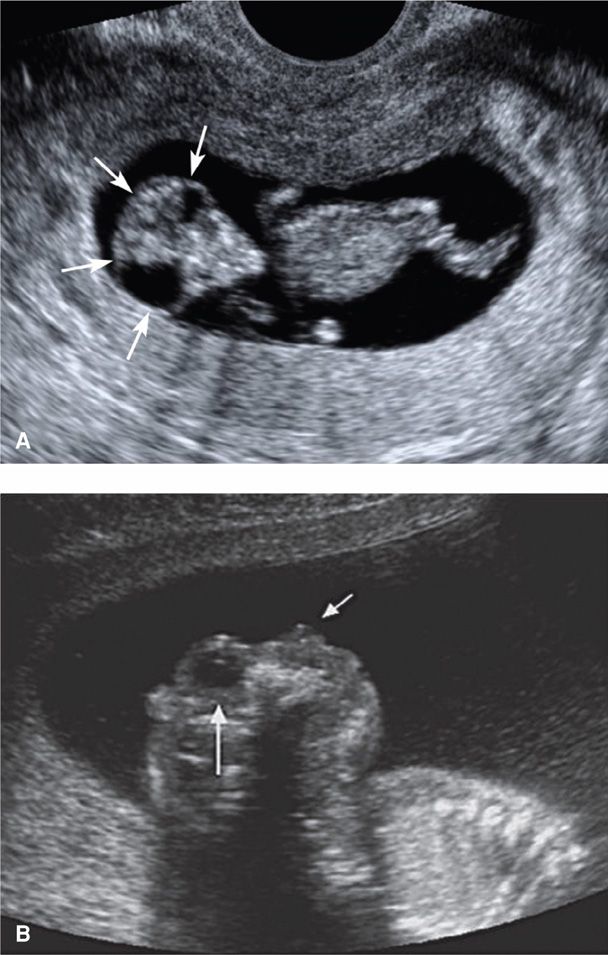
FIGURE 10-5 Anencephaly/acrania. A. Acrania. This 11-week fetus has absence of the cranium, with protrusion of a disorganized mass of brain tissue that resembles a “shower cap” (arrows) and a characteristic triangular facial appearance. B. Anencephaly. This sagittal image shows the absence of forebrain and cranium above the skull base and orbit. The long white arrow points to the fetal orbit, and the short white arrow indicates the nose.
Cephalocele is the herniation of meninges through a cranial defect, typically located in the midline occipital region (Fig. 10-6). When brain tissue herniates through the skull defect, the anomaly is termed an encephalocele. Associated hydrocephalus and microcephaly are common. Surviving infants—those with smaller defects—have a high incidence of neurological deficits and developmental impairment. Cephalocele is an important feature of the autosomal recessive Meckel-Gruber syndrome, which includes cystic renal dysplasia and polydactyly. A cephalocele not located in the occipital midline raises suspicion for amnionic-band sequence (Chap. 6, p. 121).
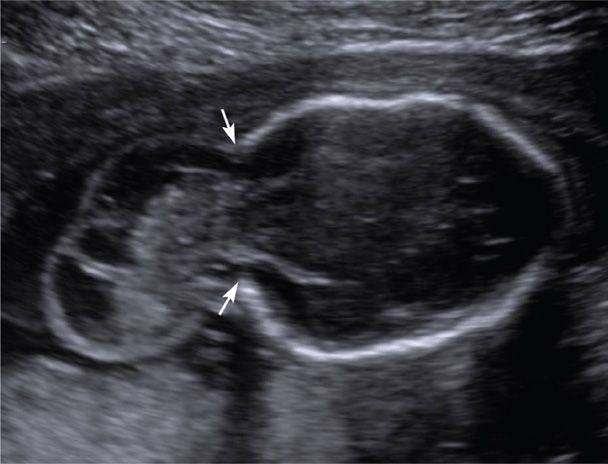
FIGURE 10-6 Encephalocele. This transverse image depicts a large defect in the occipital region of the cranium (arrows) through which meninges and brain tissue have herniated.
Spina bifida is a defect in the vertebrae, typically the dorsal arch, with exposure of the meninges and spinal cord. The birth prevalence is approximately 1 per 2000 (Cragan, 2009; Dolk, 2010). Most cases are open spina bifida—the defect includes the skin and soft tissues. Herniation of a meningeal sac containing neural elements is termed a myelomeningocele (Fig. 10-7). When only a meningeal sac is present, the defect is a meningocele. Although the sac may be easier to image in the sagittal plane, transverse images more readily demonstrate separation or splaying of the lateral processes.
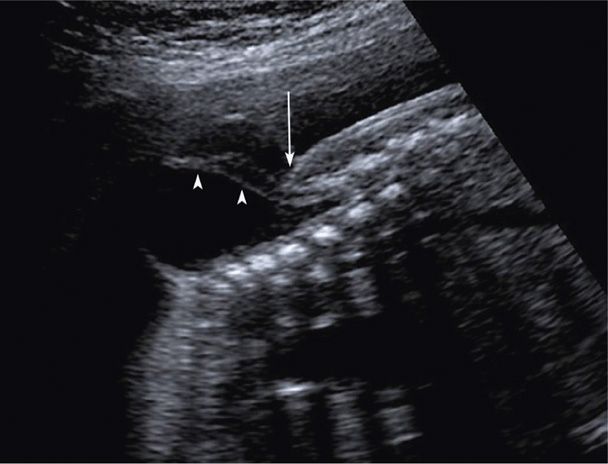
FIGURE 10-7 Myelomeningocele. In this sagittal image of a lumbosacral myelomeningocele, the arrowheads indicate nerve roots within the anechoic herniated sac. The overlying skin is visible above the level of the spinal defect but abruptly stops at the defect (arrow).
As discussed in Chapter 14 (p. 285), spina bifida may be reliably diagnosed with second-trimester sonography, often because of two characteristic cranial findings. These are scalloping of the frontal bones—the lemon sign (Fig. 14-4, p. 287), and anterior curvature of the cerebellum with effacement of the cisterna magna—the banana sign (Fig. 14-5) (Nicolaides, 1986). These findings are manifestations of the Chiari II malformation (also called Arnold-Chiari malformation), which occurs when downward displacement of the spinal cord pulls a portion of the cerebellum through the foramen magnum and into the upper cervical canal. Ventriculomegaly is another frequent sonographic finding, particularly after midgestation, and more than 80 percent of infants with open spina bifida require ventriculoperitoneal shunt placement. A small biparietal diameter is often present as well. Children with spina bifida require multidisciplinary care to address problems related to the defect, shunt, swallowing, bladder and bowel function, and ambulation. They are also at increased risk for latex allergy. Fetal surgery for myelomeningocele is discussed in Chapter 16 (p. 325).
Ventriculomegaly
This distention of the cerebral ventricles by cerebrospinal fluid (CSF) is a nonspecific marker of abnormal brain development (Pilu, 2011). The atrium normally measures between 5 and 10 mm from 15 weeks until term (see Fig. 10-2). Mild ventriculomegaly is diagnosed when the atrial width measures 10 to 15 mm, and overt or severe ventriculomegaly when it exceeds 15 mm (Fig. 10-8). CSF is produced by the choroid plexus, which is an epithelium-lined capillary core and loose connective tissue found in the ventricles. A dangling choroid plexus characteristically is found with severe ventriculomegaly.
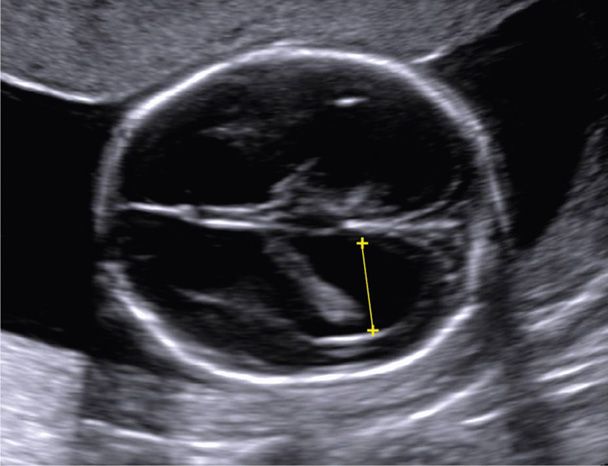
FIGURE 10-8 Ventriculomegaly. In this transverse view of the cranium, the yellow line depicts measurement of the atria, which measured 12 mm, consistent with mild ventriculomegaly.
Ventriculomegaly may be caused by various genetic and environmental insults, and prognosis is determined by etiology, degree, and rate of progression. In general, the larger the atrium, the greater the likelihood of abnormal outcome (Gaglioti, 2009; Joó, 2008). The finding of an enlarged ventricle may be due to another central nervous system abnormality—such as Dandy-Walker malformation or holoprosencephaly, due to an obstructive process—such as aqueductal stenosis, or secondary to a destructive process—such as porencephaly or an intracranial teratoma. Initial evaluation includes a specialized examination of fetal anatomy, fetal karyotyping, and testing for congenital infections such as cytomegalovirus and toxoplasmosis (Chap. 64, p. 1245).
Even when ventriculomegaly is mild and appears isolated, counseling may be a challenge because of the wide variability in prognosis. Devaseelan and colleagues (2010) conducted a systematic review of nearly 1500 pregnancies with apparently isolated mild ventriculomegaly. They found that 1 to 2 percent of cases were associated with congenital infection, 5 percent with aneuploidy, and 12 percent with neurological abnormality in the absence of infection or aneuploidy. Neurological abnormality was significantly more common if ventriculomegaly progressed, but asymmetry of ventricular size did not affect prognosis. As abnormalities associated with mild ventriculomegaly may not be detectable sonographically, fetal MR imaging may be considered.
Agenesis of the Corpus Callosum
The corpus callosum is the major fiber bundle connecting reciprocal regions of the cerebral hemispheres. With complete agenesis of the corpus callosum, a normal cavum septum pellucidum cannot be visualized sonographically, and the frontal horns are displaced laterally. Also, there is mild enlargement of the atria posteriorly—such that the ventricle has a characteristic “teardrop” appearance (Fig. 10-9). Callosal dysgenesis can also occur, in which absence involves only the caudal portions—the body and splenium—and is consequently more difficult to detect prenatally.
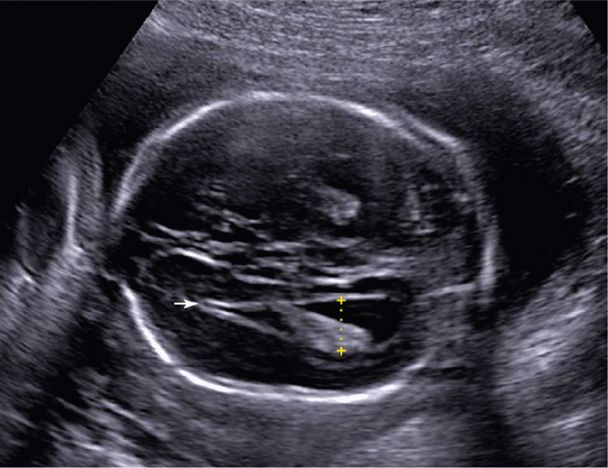
FIGURE 10-9 Agenesis of the corpus callosum. This image demonstrates a “teardrop” shaped ventricle with mild ventriculomegaly (dotted line) and laterally displaced frontal horns (arrow). A normal cavum septum pellucidum cannot be visualized.
In population-based studies, prevalence of agenesis of the corpus callosum is 1 in 5000 births (Glass, 2008; Szabo, 2011). In a recent review of apparently isolated cases of agenesis, fetal MR imaging identified additional brain abnormalities in more than 20 percent (Sotiriadis, 2012). If the anomaly was still considered isolated following MR imaging, normal developmental outcome was reported in 75 percent of cases, and severe disability in 12 percent. Agenesis of the corpus callosum is associated with other CNS and non-CNS anomalies, aneuploidy, and many genetic syndromes—more than 200—and thus genetic counseling can be challenging.
Holoprosencephaly
In early normal brain development, the prosencephalon or forebrain divides into the telencephalon and diencephalon. With holoprosencephaly, the prosencephalon fails to divide completely into two separate cerebral hemispheres and into underlying diencephalic structures. Main forms of holoprosencephaly are a continuum that contains, with decreasing severity, alobar, semilobar, and lobar types. In the most severe form—alobar holoprosencephaly—a single monoventricle, with or without a covering mantle of cortex, surrounds the fused central thalami (Fig. 10-10). In semilobar holoprosencephaly, partial separation of the hemispheres occurs. Lobar holoprosencephaly is characterized by a variable degree of fusion of frontal structures. Lobar holoprosencephaly should be considered when a normal cavum septum pellucidum cannot be visualized.
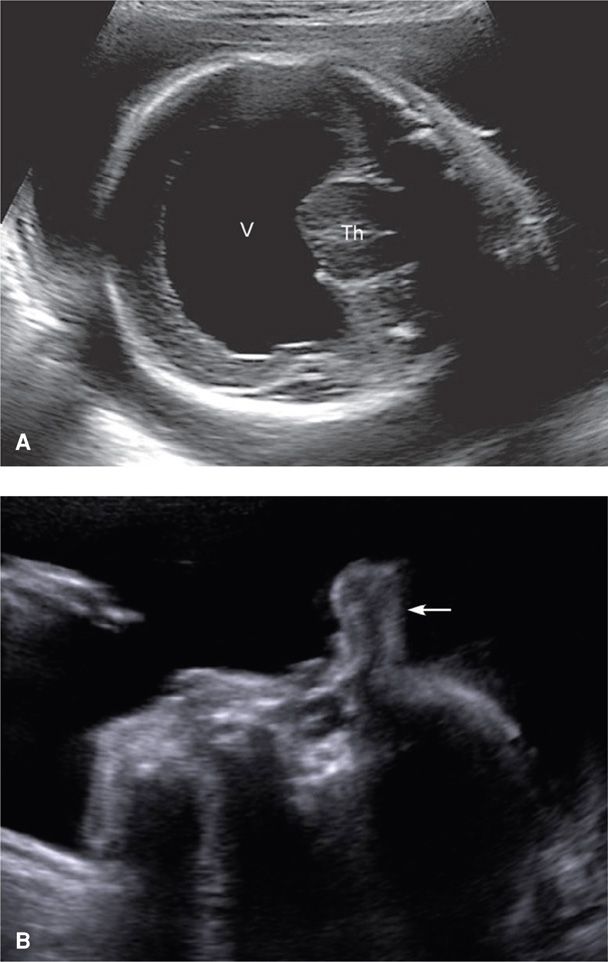
FIGURE 10-10 Alobar holoprosencephaly. A. Transverse cranial image of a 26-week fetus with alobar holoprosencephaly, depicting fused thalami (Th) encircled by a monoventricle (V). The midline falx is absent. B. In this profile view of the face and head, a soft tissue mass—a proboscis (arrow), protrudes from the region of the forehead.
Differentiation into two cerebral hemispheres is induced by prechordal mesenchyme, which is also responsible for differentiation of the midline face. Thus, holoprosencephaly may be associated with anomalies of the orbits and eyes—hypotelorism, cyclopia, or micro-ophthalmia; lips—median cleft; or nose—ethmocephaly, cebocephaly, or arhinia with proboscis (see Fig. 10-10).
The birth prevalence of holoprosencephaly is only 1 in 10,000 to 15,000. However, the abnormality has been identified in nearly 1 in 250 early abortuses, which attests to the extremely high in-utero lethality of this condition (Orioli, 2010; Yamada 2004). The alobar form accounts for 40 to 75 percent of cases, and approximately 30 to 40 percent have a numerical chromosomal abnormality, particularly trisomy 13 (Orioli, 2010; Solomon, 2010). Conversely, two thirds of trisomy 13 cases are found to have holoprosencephaly. Fetal karyotyping should be offered when this anomaly is identified.
Dandy-Walker Malformation—Vermian Agenesis
Originally described by Dandy and Blackfan (1914), this posterior fossa abnormality is characterized by agenesis of the cerebellar vermis, posterior fossa enlargement, and elevation of the tentorium. Sonographically, fluid in the enlarged cisterna magna visibly communicates with the fourth ventricle through the cerebellar vermis defect, with visible separation of the cerebellar hemispheres (Fig. 10-11). The birth prevalence is approximately 1 in 12,000 (Long, 2006). Associated anomalies and aneuploidy are very common in prenatal series. These include ventriculomegaly in 30 to 40 percent, other anomalies in approximately 50 percent, and aneuploidy in 40 percent (Ecker, 2000; Long, 2006). Dandy-Walker malformation is also associated with numerous genetic and sporadic syndromes, congenital viral infections, and teratogen exposure, all of which greatly affect the prognosis. Thus, the initial evaluation mirrors that for ventriculomegaly (p. 202).
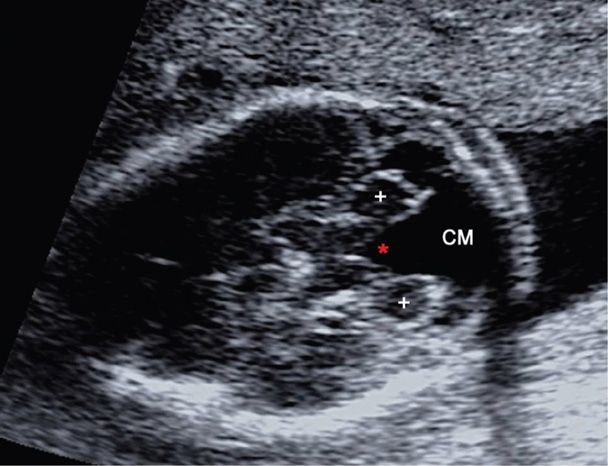
FIGURE 10-11 Dandy-Walker malformation. This transcerebellar image demonstrates agenesis of the cerebellar vermis. The cerebellar hemispheres (+) are widely separated by a fluid collection that connects the 4th ventricle (red asterisk) to the enlarged cisterna magna (CM).
Inferior vermian agenesis, also called Dandy-Walker variant, is a term used when only the inferior portion of the vermis is absent. But, even when vermian agenesis appears to be partial and relatively subtle, there is a high prevalence of associated anomalies and aneuploidy, and the prognosis is often poor (Ecker, 2000; Long, 2006).
Sacrococcygeal Teratoma
This germ cell tumor is one of the most common tumors in neonates, with a birth prevalence of approximately 1 per 28,000 (Derikx, 2006; Swamy, 2008). It is believed to arise from the totipotent cells along Hensen node, anterior to the coccyx. The American Academy of Pediatrics–Surgical Section classification for sacrococcygeal teratoma (SCT) includes four types. Type 1 is predominantly external with a minimal presacral component; type 2 is predominantly external but with a significant intrapelvic component; type 3 is predominantly internal but with abdominal extension; and type 4 is entirely internal with no external component (Altman, 1974). The tumor may be mature, immature, or malignant. Sonographically, SCT appears as a solid and/or cystic mass that arises from the anterior sacrum and usually extends inferiorly and externally as it grows (Fig. 10-12). Solid components often have varying echogenicity, appear disorganized, and may grow rapidly with advancing gestation. Internal pelvic components may be more challenging to visualize, and fetal MR imaging should be considered. Hydramnios is frequent, and hydrops may develop from high-output cardiac failure, either as a consequence of tumor vascularity or secondary to bleeding within the tumor and resultant anemia. Fetuses with tumors > 5 cm often require cesarean delivery, and classical hysterotomy may be needed (Gucciaro, 2011). Fetal surgery for SCT is discussed in Chapter 16 (p. 327).
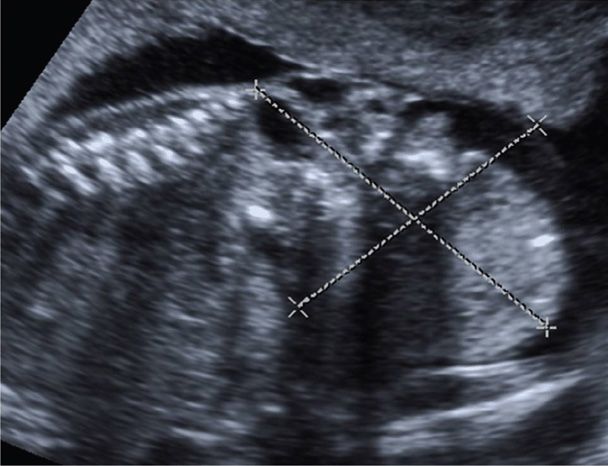
FIGURE 10-12 Sacrococcygeal teratoma. Sonographically, this tumor appears as a solid and/or cystic mass that arises from the anterior sacrum and tends to extend inferiorly and externally as it grows. In this image, a 7 × 6 cm inhomogeneous solid mass is visible below the normal-appearing sacrum. There is also an internal component to the tumor.
Caudal Regression Sequence—Sacral Agenesis
This rare anomaly is characterized by absence of the sacral spine and often portions of the lumbar spine. It is approximately 25 times more common in pregnancies with pregestational diabetes (Garne, 2012). Sonographic findings include a spine that appears abnormally short, lacks the normal lumbosacral curvature, and terminates abruptly above the level of the iliac wings. Because the sacrum does not lie between the iliac wings, they are abnormally close together and may appear “shield-like.” There may be abnormal positioning of the lower extremities and lack of normal soft tissue development. Caudal regression should be differentiated from sirenomelia, which is a rare anomaly characterized by a single fused lower extremity in the midline.
 Face and Neck
Face and Neck
Normal fetal lips and nose are shown in Figure 10-13. A fetal profile is not a required component of standard examination but may be helpful in identifying cases of micrognathia—an abnormally small jaw (Fig. 10-14). Micrognathia should be considered in the evaluation of hydramnios (Chap. 11, p. 235). Use of the ex-utero intrapartum treatment (EXIT) procedure for severe micrognathia is discussed in Chapter 16 (p. 331).
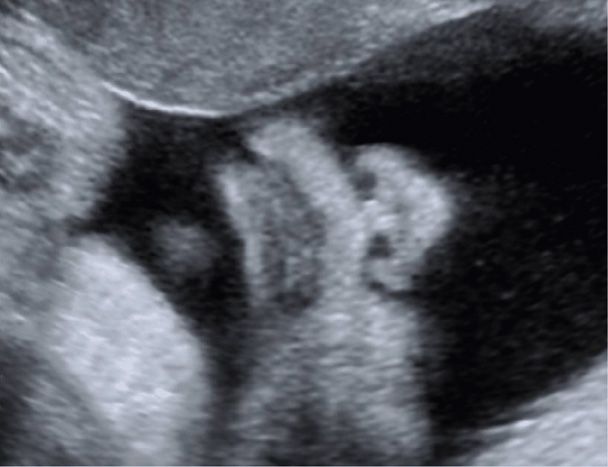
FIGURE 10-13 Midline face. This view demonstrates the integrity of the upper lip.
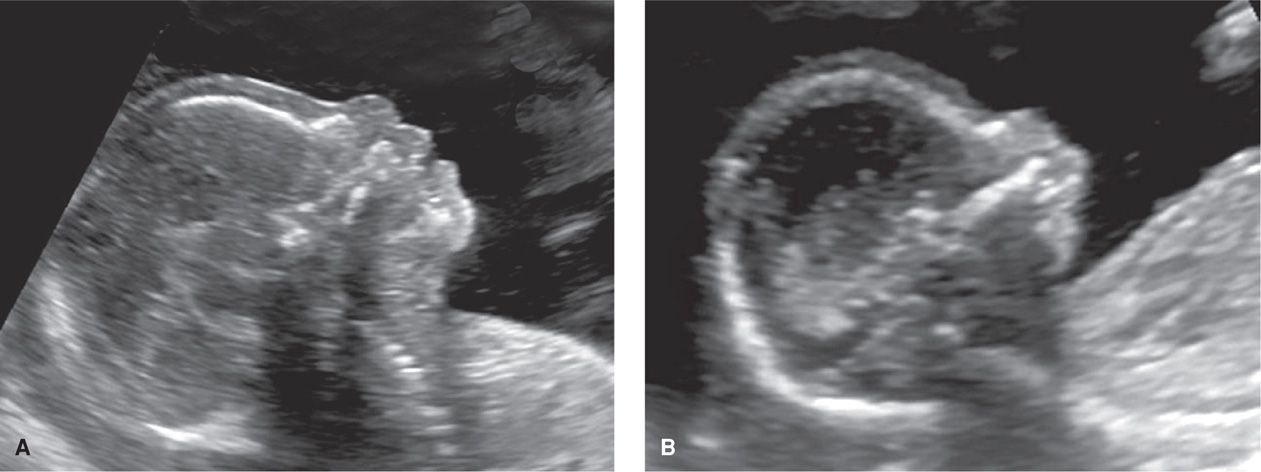
FIGURE 10-14 Fetal profile. A. This image depicts a normal fetal profile. B. This fetus has severe micrognathia, which creates a severely recessed chin.
Facial Clefts
There are three main types of clefts. The first type, cleft lip and palate, always involves the lip, may also involve the hard palate, may be unilateral or bilateral, and has a birth prevalence of approximately 1 per 1000 (Cragan, 2009; Dolk, 2010). If isolated, the inheritance is multifactorial—with a recurrence risk of 3 to 5 percent for one prior affected child. If a cleft is visible in the upper lip, a transverse image at the level of the alveolar ridge may demonstrate that the defect also involves the primary palate (Fig. 10-15).
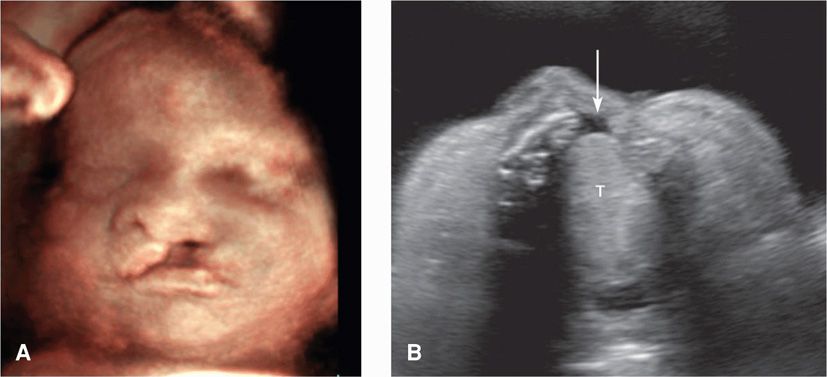
FIGURE 10-15 Cleft lip/palate. A. This fetus has a prominent unilateral (left-sided) cleft lip. B. Transverse view of the palate in the same fetus demonstrates a defect in the alveolar ridge (arrow). The tongue (T) is also visible.
In a recent systematic review of low-risk pregnancies, cleft lip was identified sonographically in only about half of cases (Maarse, 2010). Approximately 40 percent of those detected in prenatal series are associated with other anomalies or syndromes, and aneuploidy is common (Maarse, 2011; Offerdal, 2008). The rate of associated anomalies is highest for bilateral defects that involve the palate. Using data from the Utah Birth Defect Network, Walker and associates (2001) identified aneuploidy in 1 percent with cleft lip alone, 5 percent with unilateral cleft lip and palate, and 13 percent with bilateral cleft lip and palate. It seems reasonable to offer fetal karyotyping when a cleft is identified.
The second type of cleft is isolated cleft palate. It begins at the uvula, may involve the soft palate, and occasionally involves the hard palate—but does not involve the lip. The birth prevalence is approximately 1 per 2000 (Dolk, 2010). Identification of isolated cleft palate has been described using specialized 2- and 3-dimensional sonography (Ramos, 2010; Wilhelm, 2010). However, it is not expected to be visualized during a standard ultrasound examination (Maarse, 2011; Offerdal, 2008).
A third type of cleft is median cleft lip, which is found in association with several conditions. These include agenesis of the primary palate, hypotelorism, and holoprosencephaly. Median clefts may also be associated with hypertelorism and frontonasal hyperplasia, formerly called the median cleft face syndrome.
Cystic Hygroma
This is a venolymphatic malformation in which fluid-filled sacs extend from the posterior neck (Fig. 10-16). Cystic hygromas may be diagnosed as early as the first trimester and vary widely in size. They are believed to develop when lymph from the head fails to drain into the jugular vein and accumulates instead in jugular lymphatic sacs. Their birth prevalence is approximately 1 in 5000, but given the high in-utero lethality of this condition, the incidence is much higher (Cragan, 2009).
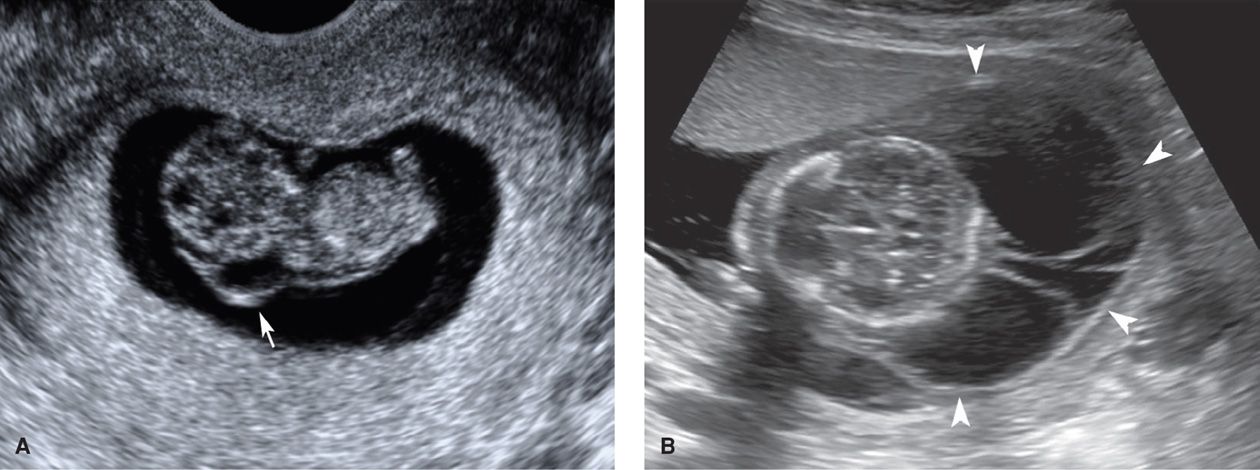
FIGURE 10-16 Cystic hygromas. A. This 9-week fetus with a cystic hygroma (arrow) was later found to have Noonan syndrome. B. Massive multiseptated hygromas (arrowheads) in the setting of hydrops fetalis at 15 weeks.
Up to 70 percent of cystic hygromas are associated with aneuploidy. Of those diagnosed in the second trimester, approximately 75 percent of aneuploid cases are 45,X—Turner syndrome (Johnson, 1993; Shulman, 1992). When cystic hygromas are diagnosed in the first trimester, trisomy 21 and 45,X are both common, followed by trisomy 18 in frequency (Kharrat, 2006; Malone, 2005). In a review of more than 100 cystic hygroma cases from the First And Second Trimester Evaluation of Risk (FASTER) trial, trisomy 21 was the single most common aneuploidy (Malone, 2005). First-trimester fetuses with cystic hygromas were five times more likely to be aneuploid than the fetuses with an increased nuchal translucency.
In the absence of aneuploidy, cystic hygromas confer a significantly increased risk for other anomalies, particularly cardiac anomalies that are flow-related. These include hypoplastic left heart and coarctation of the aorta. Cystic hygromas also may be part of a genetic syndrome. One is Noonan syndrome, an autosomal dominant disorder that shares several features with Turner syndrome, including short stature, lymphedema, high-arched palate, and often pulmonary valve stenosis.
Large cystic hygromas are usually found with hydrops fetalis, rarely resolve, and carry a poor prognosis. Small hygromas may undergo spontaneous resolution, and provided that fetal karyotype and echocardiography results are normal, the prognosis may be good. The likelihood of a nonanomalous liveborn infant with normal karyotype following identification of first-trimester hygroma is approximately 1 in 6 (Kharrat, 2006; Malone, 2005).
 Thorax
Thorax
The lungs appear as homogeneous structures surrounding the heart and are best visualized after 20 to 25 weeks’ gestation. In the four-chamber view of the chest, they comprise approximately two thirds of the area, with the heart occupying the remaining third. The thoracic circumference is measured at the skin line in a transverse plane at the level of the four-chamber view. In cases of suspected pulmonary hypoplasia secondary to a small thorax, such as with severe skeletal dysplasia, comparison with a reference table may be helpful (Appendix, p. 1299). Various abnormalities may be seen sonographically as cystic or solid space-occupying lesions. Fetal therapy for thoracic abnormalities is discussed in Chapter 16 (p. 329).
Congenital Diaphragmatic Hernia
This is a defect in the diaphragm through which abdominal organs herniate into the thorax. It is left-sided in approximately 75 percent of cases, right-sided in 20 percent, and bilateral in 5 percent (Gallot, 2007). The prevalence of congenital diaphragmatic hernia (CDH) is approximately 1 per 3000 to 4000 births (Cragan, 2009; Dolk, 2010; Gallot, 2007). Associated anomalies and aneuploidy occur in 40 percent of cases (Gallot, 2007; Stege, 2003). Targeted sonography and fetal echocardiography should be performed, and fetal karyotyping should be offered. Given the association with genetic syndromes, chromosomal microarray analysis is a consideration (Chap. 13, p. 277). In population-based series, the presence of an associated abnormality reduces the overall survival rate of neonates with diaphragmatic hernia from approximately 50 percent to about 20 percent (Colvin, 2005; Gallot, 2007; Stege, 2003). In the absence of associated abnormalities, the major causes of mortality are pulmonary hypoplasia and pulmonary hypertension.
Sonographically, the most frequent finding with a left-sided defect is repositioning of the heart to the mid or right hemithorax. With this, the axis of the heart points toward the midline (Fig. 10-17). Associated findings include the stomach bubble or bowel peristalsis in the chest and a wedge-shaped mass—the liver—located anteriorly in the left hemithorax. Liver herniation complicates at least 50 percent of cases and is associated with a 30-percent reduction in the survival rate (Mullassery, 2010). With large lesions, impaired swallowing and mediastinal shift may result in hydramnios and hydrops, respectively.
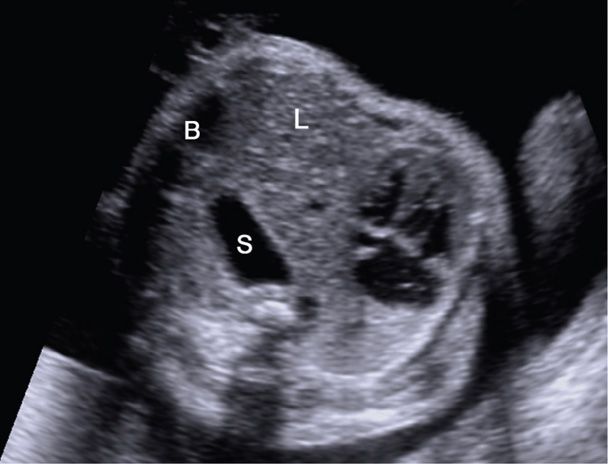
FIGURE 10-17 Congenital diaphragmatic hernia. In this transverse view of the thorax, the heart is shifted to the far right side of the chest by a left-sided diaphragmatic hernia containing stomach (S), liver (L), and bowel (B).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


