Arrhythmias
George F. Van Hare
GENERAL PRINCIPLES
 ANATOMY AND PHYSIOLOGY
ANATOMY AND PHYSIOLOGY
The heart has specialized cells collected into nodes and tracts. The sinoatrial node, near the junction of the superior vena cava and right atrium, has a rich vagal and sympathetic nerve supply and controls heart rate. Conduction of impulses from the sinoatrial node to the atrioventricular node occurs without a specialized conducting system.  The slow cell-to-cell conduction through atrial myocardium explains the relatively long duration of the P wave.
The slow cell-to-cell conduction through atrial myocardium explains the relatively long duration of the P wave.
The atrioventricular (AV) node is in the interatrial septum just anterior and superior to the mouth of the coronary sinus; it is also innervated by vagal and sympathetic fibers and consists of a mesh of very thin fibers that conduct impulses very slowly. As a result, the AV node delays conduction, giving time for ventricular filling. Also, in the presence of atrial fibrillation, the AV node limits the number of impulses reaching the ventricles. From the AV node, the bundle of His passes through the central fibrous body into the ventricular septum just behind and below its membranous portion.1 Near the summit of the muscular ventricular septum, the bundle of His gives off the compact right bundle branch with wide fibers and then the left branch bundle with a diffuse fan of thinner fibers. The bundle is insulated from surrounding myocardium and normally does not activate the ventricular myocardium until it branches and ramifies into the Purkinje fibers. These peripheral conducting fibers ramify just beneath the endocardium so that the ventricular walls are depolarized from subendocardium to subepicardium. Rapid conduction down the His-Purkinje system allows the entire ventricular myocardium to contract nearly simultaneously, explaining the narrow QRS complex in normal hearts.
Some people have accessory pathways connecting atrial and ventricular myocardium. In patients with Wolff-Parkinson-White syndrome, the bundle of Kent is a muscular bridge spanning the atrioventricular groove.2 With some Kent bundles, conduction is possible in both anterograde and retrograde directions; in others, it is exclusively retrograde. Anterograde conduction causes early depolarization of the ventricles (preexcitation); retrograde conduction causes rapid reentry between the atria and the ventricles, causing sustained tachyarrhythmias. A second type of accessory pathway, the Mahaim fiber, is thought to be made up of specialized conducting fibers and to connect directly to the specialized conducting system.3 The most common type, called the atriofascicular connection, connects atrial myocardium with elements of the right bundle branch. In general, only anterograde conduction is thought to be possible in Mahaim pathways; tachycardia occurs as a result of anterograde conduction from the atrium to the distal conducting system and then retrograde conduction to the bundle of His, atrioventricular node, and back to the atrium.
Some individuals have dual atrioventricular (AV) node pathways.4 These pathways are functionally and anatomically distinct, with the atrial approaches to the slow pathway being posterior and inferior to the compact AV node and to the fast pathway being anterior and superior to the node. The effective refractory period is usually shorter in the slow pathway than in the fast. Dual AV node pathways provide the basis for atrioventricular node reentry tachycardia; after a premature atrial contraction blocks in the fast pathway and conducts down the slow, the impulse may reenter the fast pathway retrograde, setting up sustained reentrant tachycardia.
For a detailed discussion of cardiac cellular electrophysiology see DVD text. 
BASIC FEATURES OF ARRHYTHMIAS
There are 3 major types of disorders: abnormal automaticity (impulse formation), abnormal conduction, and fibrillation.
 DISORDERS OF AUTOMATICITY
DISORDERS OF AUTOMATICITY
If the sinoatrial node discharges abnormally slowly or the impulse from it is not conducted, an ectopic lower pacemaker, either atrial or more commonly junctional, takes over. If the block is in the atrioventricular node or bundle of His, a ventricular pacemaker may take over. In this way, lower pacemakers escape from suppression by higher pacemakers. Escape may occur for a single beat, for a few beats, or may result in a permanent lower pacemaker rhythm if the higher pacemaker does not regain control. Escape beats or rhythms indicate abnormality of the higher pacemaker or abnormal conduction from it, and the escape is a normal response. An escape beat is recognized by its late appearance (R-R interval longer than normal) and evidence of an ectopic focus (abnormal P-wave axis and morphology for an atrial ectopic focus; no P wave, very short PR; or retrograde P wave for junctional focus). An escape rhythm is characterized by an ectopic rhythm that is slower than a normal sinus rhythm (eFig. 485.2  ).
).
The other disorder of automaticity is premature discharge of a lower pacemaker. One or 2 of these beats are termed premature beats or extrasystoles, but 3 or more in a row constitute ectopic tachycardia. If the ectopic focus is in the atrium or is junctional, the descending impulse reaches the ventricles through the His-Purkinje system, and a normal QRS complex usually occurs (but see Aberrant Conduction, below). If the ectopic focus is ventricular, the impulse spreads slowly through muscle cells and produces a widened, bizarre QRS-T complex with the polarities of the QRS complex and the T wave in opposition to each other. If the premature beat begins in the right ventricle, then this ventricle is depolarized before the left ventricle and the QRS pattern resembles a left bundle branch block. Conversely, a left ventricular ectopic beat resembles a right bundle branch block. The basic difference between bundle branch block and ectopic patterns is that in the former only the terminal part of depolarization is slow, whereas in the latter the whole QRS complex is widened and distorted. It may be difficult to make this differentiation in practice. However, in children, most aberrantly conducted beats with bundle branch block follow a premature atrial beat. Therefore, a wide QRS complex without a discernable premature P wave is likely to be ventricular in origin.
With a ventricular premature beat, the retrograde impulse is often blocked at the atrioventricular node and the basic rhythm of the sinoatrial node is usually unaltered, thus the first sinus beat after a ventricular extrasystole follows a full compensatory pause. That is, the interval between the sinus beat preceding the extrasystole and the sinus beat following it is at least twice the normal R-R interval (Fig. 485-1). However, with atrial or junctional premature beats, the sinoatrial node is usually discharged; thus, the next sinus beat usually occurs after an incomplete compensatory pause (less than twice the normal R-R interval).
Ectopic tachycardias have QRS complexes like those of the corresponding ectopic beats: usually normal if the focus is supraventricular, almost always widened if it is ventricular, but widened with a supraventricular focus and aberrant conduction. These are differentiated below.
The effect of premature atrial beats on P-wave morphology depends on the site of the atrial focus. If it is near the sinoatrial node, an almost normal P wave occurs (eFig. 485.3  ); if it is near the atrioventricular (AV) node, then the atrial axis is directed superiorly, leading to inverted P waves in leads II, III, and aVF (Fig. 485-1B). Therefore, low atrial and high His-bundle premature beats give similar P waves, although, because of the time taken for retrograde conduction through the AV node to the atria, an infranodal pacemaker is more likely to give P waves buried in the QRS complex or following it. If the ectopic focus is ventricular, there can be an abnormal P wave after the QRS complex due to retrograde AV node conduction, but often the retrograde impulse is blocked in the AV node and the atrium is activated normally from the sinoatrial node at a different rate.
); if it is near the atrioventricular (AV) node, then the atrial axis is directed superiorly, leading to inverted P waves in leads II, III, and aVF (Fig. 485-1B). Therefore, low atrial and high His-bundle premature beats give similar P waves, although, because of the time taken for retrograde conduction through the AV node to the atria, an infranodal pacemaker is more likely to give P waves buried in the QRS complex or following it. If the ectopic focus is ventricular, there can be an abnormal P wave after the QRS complex due to retrograde AV node conduction, but often the retrograde impulse is blocked in the AV node and the atrium is activated normally from the sinoatrial node at a different rate. 
Tachycardias may also result from reentry, a circular form of depolarization that occurs when there are 2 parallel pathways with unidirectional block and often a protected zone of slow conduction. For example, consider a strip of damaged ventricular muscle that does not conduct anterograde, but does conduct slowly retrograde. The normal sinus impulse descends and depolarizes the normal, but not the abnormal, muscle. The wave of depolarization eventually passes slowly retrograde up the abnormal strip that has not been depolarized; by the time the impulse emerges from the abnormal muscle, the rest of the muscle is responsive and is depolarized prematurely—a ventricular premature beat. Usually, the wave of depolarization does not continue to circulate because the abnormal muscle, being depolarized, is then refractory to impulses from any direction. The sequence may be repeated to produce ventricular bigeminy. Because normally there is slow conduction through the atrioventricular node, reentry through it is believed to explain many types of extrasystoles. If the recirculating wave of depolarization is itself slowed in some other portion of muscle, then the wave may return to the original abnormal muscle strip when it has regained excitability, and a rapid repetitive depolarization (reentry tachycardia) will occur. Reentry is the cause of tachycardia in Wolff-Parkinson-White syndrome, in which a premature atrial impulse blocks in the accessory pathway, but descends through the slowly conducting atrioventricular node, returns to the atrium through the rapidly conducting bundle of Kent, and continues around the circuit in an endless loop.
 DISORDERS OF CONDUCTION
DISORDERS OF CONDUCTION
Conduction may be impaired either by an abnormal increase in the refractory period (pathologic or primary) or by interference from another impulse that alters the refractory period (physiologic or secondary). Physiologic factors that affect conduction include concealed conduction and physiological interference. When conduction through tissue cannot be detected on the electrocardiogram but is inferred by its effect on conduction of a subsequent impulse, then it is termed concealed conduction. 
Interference is shown most simply if there is a very early isolated atrial premature beat. In eFigure 485.3  , the ninth P wave is seen on the T wave of the preceding QRS complex, but because the AV node or ventricle is still refractory, the impulse is not conducted. This is a physiologic, not pathologic, failure of conduction and so does not imply any abnormality in the AV node. A more prolonged episode of physiologic interference may occur if the sinus pacemaker slows or a junctional pacemaker speeds up so that the atria and ventricles beat at roughly similar rates but are controlled by different pacemakers (see eFigs. 485.1
, the ninth P wave is seen on the T wave of the preceding QRS complex, but because the AV node or ventricle is still refractory, the impulse is not conducted. This is a physiologic, not pathologic, failure of conduction and so does not imply any abnormality in the AV node. A more prolonged episode of physiologic interference may occur if the sinus pacemaker slows or a junctional pacemaker speeds up so that the atria and ventricles beat at roughly similar rates but are controlled by different pacemakers (see eFigs. 485.1  and 485.5
and 485.5  ). This is one form of atrioventricular dissociation (the other main form being complete atrioventricular block). Despite a normal AV conduction system, the descending impulse from the sinoatrial node does not pass the AV node, which has been made refractory by the retrograde impulse from the ventricle or bundle of His. In turn, this retrograde impulse does not capture the atrium, which has been made refractory by the sinus beat. Failure of the sinoatrial node to capture the ventricle is thus not an abnormality of conduction but a physiologic effect on refractory periods. An electrocardiogram with this type of interference shows unrelated P waves and QRS complexes with the ventricular rate faster than the atrial rate. Occasionally, ventricular and atrial rates are the same, but the PR interval is too short to diagnose a sinus rhythm; this is termed isorhythmic atrioventricular dissociation.
). This is one form of atrioventricular dissociation (the other main form being complete atrioventricular block). Despite a normal AV conduction system, the descending impulse from the sinoatrial node does not pass the AV node, which has been made refractory by the retrograde impulse from the ventricle or bundle of His. In turn, this retrograde impulse does not capture the atrium, which has been made refractory by the sinus beat. Failure of the sinoatrial node to capture the ventricle is thus not an abnormality of conduction but a physiologic effect on refractory periods. An electrocardiogram with this type of interference shows unrelated P waves and QRS complexes with the ventricular rate faster than the atrial rate. Occasionally, ventricular and atrial rates are the same, but the PR interval is too short to diagnose a sinus rhythm; this is termed isorhythmic atrioventricular dissociation. 
 ABERRANT CONDUCTION
ABERRANT CONDUCTION
Whenever a supraventricular impulse reaches the atrioventricular (AV) node or bundle of His in the relative refractory period, the impulse may be transmitted aberrantly because of uneven loss of refractoriness in the AV node or bundle branches. The impulse may arrive at the AV node or bundle at this time because of a premature supraventricular beat, supraventricular tachycardia, or a prolonged QT interval of the preceding beat. In most people, the right bundle branch normally has a longer refractory period than the left bundle. Therefore, premature supraventricular beats or tachycardias may be conducted normally to the left ventricle but slowly to the right ventricle, giving the pattern of right bundle branch block and a wide QRS complex (eFig. 485.3  ). However, left bundle aberration occurs occasionally. Bundle branch aberration after a single premature supraventricular beat is quite common, particularly if the beat follows a long pause (Ashman phenomenon). However, sustained bundle branch aberration with supraventricular tachycardia is less common in children than adults, and most sustained wide QRS tachycardias in children are caused by ventricular tachycardia.
). However, left bundle aberration occurs occasionally. Bundle branch aberration after a single premature supraventricular beat is quite common, particularly if the beat follows a long pause (Ashman phenomenon). However, sustained bundle branch aberration with supraventricular tachycardia is less common in children than adults, and most sustained wide QRS tachycardias in children are caused by ventricular tachycardia.
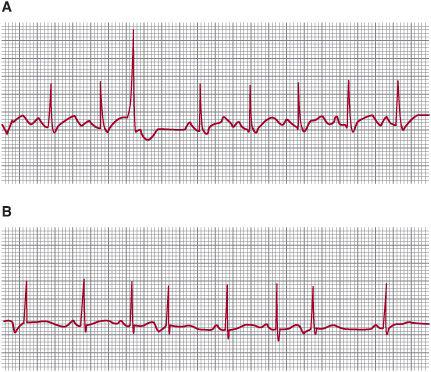
FIGURE 485-1. Compensatory pauses following ectopic beats. A: Premature ventricular ectopic beat followed by a full compensatory pause. B: Premature atrial contractions with incomplete and full compensatory pauses. Both strips are lead II.
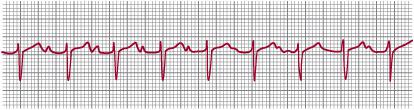
FIGURE 485-2. First-degree atrioventricular block in a newborn infant. The P-R interval is 0.18 seconds, which is prolonged for the patient’s age and heart rate.
An important form of aberration occurs when the ventricle during tachycardia is activated entirely or in part from an accessory AV connection. Activation from a Kent bundle does not use the His-Purkinje system, thus conduction through the ventricle is slow, as with a ventricular ectopic focus. This phenomenon is seen in Wolff-Parkinson-White syndrome with atrial tachycardia or fibrillation or in antidromic reentry with conduction anterograde in the pathway and retrograde in the AV node. It is also seen during reentry involving a Mahaim atriofascicular connection, in which conduction is down the pathway to the right bundle branch with retrograde activation to the atrium via the bundle of His and AV node.
 ATRIOVENTRICULAR BLOCK
ATRIOVENTRICULAR BLOCK
If conduction is delayed through the atrioventricular (AV) node or the bundle of His, then various degrees of AV block occur. If there is merely a long PR interval but all beats are conducted, first-degree AV block is diagnosed (Fig. 485-2). If no atrial beats are conducted so that the ventricles are driven by a junctional or ventricular focus, then there are normal P waves at one rate and QRS complexes at a slower rate, usually with no fixed relationship between P waves and QRS complexes. This is complete or third-degree AV block (Fig. 485-3).
Whenever atria and ventricles are controlled by independent pacemakers, there is AV dissociation. This may result either from complete AV block or from junctional or ventricular tachycardia or accelerated rhythm without retrograde conduction. The former has faster atrial than ventricular rates; the descending impulse cannot reach the lower pacemaker because a conduction block exists. The latter has faster ventricular than atrial rates, but coexistent anterograde conduction block is not excluded. The absence of anterograde conduction block is demonstrated when a critically timed atrial impulse conducts to the ventricles and suddenly shortens the R-R interval. In ventricular tachycardia, this shortened R-R interval is associated with shortening of, or normalization of, the QRS complex. In junctional tachycardia, the QRS remains relatively unchanged in capture beats. If there is doubt about AV conduction, one can determine if block is present by increasing atrial rate with exercise or hyperventilation and noting whether or not ventricular capture occurs (eFig. 485.5  ).
).
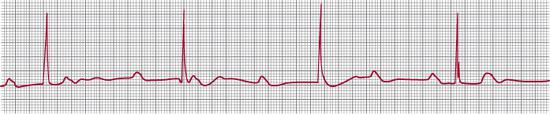
FIGURE 485-3. Third-degree atrioventricular block. The P waves and QRS complexes have no consistent temporal relationship. The atrial rate is 90 beats per minute, and the ventricular 38 beats per minute.
If some sinus beats are conducted to the ventricle but others do not reach it, there is second-degree atrioventricular (AV) block. Mobitz type I second-degree AV block is also known as Wenckebach conduction and is generally due to failure of conduction in the AV node. The first atrial impulse of a group of beats is conducted normally, but the next atrial impulse reaches the AV node while it is still partly refractory and thus is conducted more slowly, giving a longer PR interval. The next atrial impulse arrives even earlier in the AV nodal refractory period, with an even longer PR interval as a result. Eventually the atrial impulse reaches the AV node in its absolute refractory period and is blocked so that no QRS complex follows (Fig. 485-4). 
Mobitz type II second-degree atrioventricular (AV) block occurs when a QRS complex drops out without prior lengthening of the PR intervals and usually occurs distal to the AV node. It is less common but more serious than type I second-degree AV block and is more likely to lead to complete AV block. 
 ATRIAL AND VENTRICULAR FIBRILLATION
ATRIAL AND VENTRICULAR FIBRILLATION
Sometimes a stimulus to a region that has repolarized is conducted at different rates in some directions and blocked in others. As a result, an irregular wave front of depolarization takes a tortuous path through the muscle and leaves behind it an irregular path of refractory cells. Portions of the wave front return to regions that were formerly refractory but are now excitable. The wave of depolarization becomes more irregular, and eventually there is chaotic fragmented contraction that is termed fibrillation. 
If the chaotic rhythm occurs in the atria, there is atrial fibrillation, which is easily initiated by sustained rapid atrial pacing. However, in children, atrial fibrillation is usually not sustained and reverts to sinus rhythm unless the atria are enlarged. If the chaotic rhythm occurs in the ventricles, there is ventricular fibrillation: The vulnerable period in which a shock (or rarely, an ectopic beat) can cause fibrillation is at the apex of the T wave.
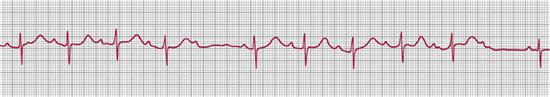
FIGURE 485-4. Second-degree atrioventricular block. The tracing shows 2 Wenckebach cycles with progressive lengthening of the P-R interval until the atrial beat is not conducted to the ventricles.
DIAGNOSIS
Arrhythmias are best diagnosed with knowledge of the history and physical findings and with all previous electrocardiograms from that patient. The electrocardiogram of the arrhythmia under study should be a routine full electrocardiogram plus some very long strips, lasting about 1 minute or more. In sinus rhythm, leads II and V1 are most likely to show good P waves, but with ectopic atrial foci, other leads may be better. Sometimes special maneuvers are needed to bring out P waves, such as using modified chest, esophageal, or intracardiac leads.
The electrocardiogram should be examined carefully for P waves. These should be inspected for variations in rate, morphology, and mean axis, for PR intervals and their possible variation, and to find out if the P waves are likely to be the source of the impulse causing the QRS complex. Then, the QRS complex should be examined for its rate, rhythm, and morphology.
Maneuvers that increase atrioventricular block (eg, vagal stimuli, certain drugs such as adenosine) may slow ventricular rate and allow better definition of atrial and ventricular complexes. They should be performed only while the electrocardiogram is running, for safety as well as for future analysis.
Most arrhythmias can be diagnosed by careful diagnosis of the electrocardiogram during the occurrence of the arrhythmia as well as in the baseline state. Often, however, additional methods are helpful. Recordings of paroxysmal arrhythmias are often difficult to obtain, particularly at their onset or termination, which are the most important features for analysis. If episodes occur daily, a 24-hour ambulatory electrocardiogram (Holter monitor) may capture the episode. If they are less frequent, transtelephonic event recorders, with or without looping memory, that are activated by the patient when symptoms occur may capture an episode. Another option is surgically implanted loop recorder that has a battery life of up to several years.5 Sometimes tachycardias are precipitated by exercise, and an exercise test may elicit the arrhythmia. Invasive electrophysiologic study is the definitive method of diagnosing cardiac arrhythmias. As many as 4 electrode catheters are inserted via veins into the heart for pacing and recording the intracardiac electrograms. Both sinoatrial and atrioventricular node function are assessed, and arrhythmias may be induced, diagnosed, and mapped to determine their origin and route of conduction by programmed electrical stimulation.
 ARRHYTHMIAS WITH REGULAR RHYTHM AT NORMAL RATE
ARRHYTHMIAS WITH REGULAR RHYTHM AT NORMAL RATE
A regular rhythm at normal rate is most likely to be sinus rhythm, but the electrocardiogram should be inspected carefully to exclude atrial tachycardia with 2:1 atrioventricular block, atrial flutter with 4:1 atrioventricular block, accelerated junctional or idioventricular rhythms, or atrial fibrillation with an independent idioventricular rhythm caused by a coexistent atrioventricular block.
 PAUSES
PAUSES
Occasional pauses are most commonly caused by nonconducted very early atrial extrasystoles; these may not always be readily apparent, as the P waves may be superimposed on the preceding T wave. They can also result from marked sinus arrhythmia, second-degree atrioventricular block, or sinoatrial exit block as well as concealed conduction of junctional extrasystoles that have made the atrioventricular node refractory.
 GROUPS OF BEATS
GROUPS OF BEATS
Bigeminy means that beats occur in pairs. Most commonly this is because of extrasystole coupled to the previous normal beat, but it can also indicate a 3:2 atrioventricular block (every third atrial beat not conducted) or an escape beat paired with the next normal beat. Similarly, trigeminy means groups of 3 beats. These could be 2 normal beats and 1 premature beat, 1 normal and 2 premature beats, 4:3 atrioventricular block, and so on. Wenckebach periods often produce beats that are grouped in pairs, triplets, or larger groups.
 IRREGULAR RHYTHMS
IRREGULAR RHYTHMS
Occasional irregularities result from premature beats or escape beats, the latter occurring with sinus pauses, blocked atrial premature beats, or second-degree atrioventricular block. These are usually easy to differentiate. At times it is difficult to distinguish atrial fibrillation from a second-degree atrioventricular block with Wenckebach periods occurring in a tachycardia, where P waves are not easy to find. The diagnosis is made by the characteristic features of Wenckebach periods: progressive shortening of the R-R interval before a long pause, with the longest R-R interval being less than 2 of the shortest intervals.
 TACHYCARDIAS
TACHYCARDIAS
A rapid heart rate with normal P waves and PR intervals, and with variations in rate from moment to moment, is a sinus tachycardia. A rapid fixed rate with complete regularity, normal QRS complexes, and either P waves on the T waves or else no clear P waves is probably a supraventricular tachycardia; vagal stimulation causes either no change or an abrupt reversion to sinus rhythm. A rapid rate, usually fixed and regular, with a wide QRS complex is either a ventricular tachycardia or a supraventricular tachycardia with ventricular aberration or preceding bundle branch block. A ventricular tachycardia can be diagnosed by examining the QRS patterns (see above), by finding fusion beats, or by noting that the QRS complexes are similar to ventricular ectopic beats found in other electrocardiograms. Vagal stimulation usually does not affect this arrhythmia. If a slower atrial rhythm is noted from P waves usually deforming the T waves variously from beat to beat, then ventricular tachycardia is likely. However, retrograde P waves at the same rate as the ventricles can occur with junctional or ventricular tachycardias. A rapid supraventricular tachycardia can also be caused by atrial flutter with a regular block; this is differentiated from a plain supraventricular tachycardia by the typical sawtooth flutter waves, although these waves may be seen in only 1 or 2 leads. It could also result from atrial fibrillation, detected by slight variations in the R-R intervals. In both these arrhythmias the rate is slowed and the variability is increased by increasing atrioventricular nodal block with vagal stimulation or digitalis.
TREATMENT
 PRINCIPLES OF TREATMENT
PRINCIPLES OF TREATMENT
In addition to correctly identifying the arrhythmia, proper management of arrhythmias requires attention to 4 questions.
1. Does the arrhythmia need treatment? In many children no treatment is needed. Many antiarrhythmic agents may cause new, or worsen existing, arrhythmias or even cause sudden death. Arrhythmias that require treatment are those that threaten to cause ventricular fibrillation or asystole (eg, ventricular tachycardia, multiform ventricular ectopic beats) and those in which the ventricular rate is too slow or too fast for effective cardiac output. In general, the effects of abnormal ventricular rates depend not only on the rates but also on how much they differ from the patient’s usual rate. Thus, a chronically slow ventricular rate of 50 beats per minute is accompanied by ventricular dilatation and hypertrophy and well-maintained cardiac output. On the other hand, a sudden drop from 100 to 50 beats per minute may be poorly tolerated.
2. Can underlying causes be corrected? In children, particularly neonates, most clinically significant arrhythmias are caused by apnea, hypoxemia, acidemia, electrolyte disturbances, or drugs such as digitalis or catecholamines. Acute digoxin toxicity can cause life-threatening arrhythmias that are best treated with digoxin immune Fab.6 If the arrhythmia is treated without attention to the underlying cause, the patient may deteriorate.
3. Do secondary effects need treatment? Arrhythmias that reduce cardiac output may cause hypotension and acidemia, with subsequent increase in sympathetic tone, which helps to maintain the arrhythmia.
4. What specific therapeutic strategy is needed for the arrhythmia? Consider 2 patients with 2:1 atrioventricular block, 1 with an atrial rate of 260 beats per minute, the other with an atrial rate of 100 beats per minute. The first patient has an adequate ventricular rate of 130 beats per minute, and therapy should be directed at slowing the atrial rate; the atrioventricular block should not be treated because it is useful in preventing too many impulses from reaching the ventricles and causing a harmful rapid ventricular rate. In the second patient, therapy should be directed to increasing the ventricular rate of 50 beats per minute by improving atrioventricular conduction or by pacing the ventricles.
 PHARMACOLOGIC MANAGEMENT
PHARMACOLOGIC MANAGEMENT
Methods of controlling arrhythmias may be chosen with the mechanism of the specific arrhythmia in mind. However, caution is needed when extrapolating pharmacologic effects observed in normal cardiac muscle to diseased muscle. For example, in ischemic heart muscle, lidocaine prolongs conduction and refractoriness, whereas it increases conduction velocity in normal fibers; in fact, its effectiveness against some ventricular arrhythmias may depend on this action (blocking reentry) rather than on its depression of excitability. For this reason, selection of the appropriate antiarrhythmic agent should be based on clinical experience as well as theory or the effects in single cardiac cells.
Bradycardias
A slow ventricular rate may be caused by severe sinus bradycardia or arrest, or second- or third-degree atrioventricular (AV) block. The slowing either causes a cardiac output that is too low or allows breakthrough of potentially dangerous ventricular escape beats or tachycardia. Treatment is aimed at increasing the ventricular rate. Atropine (0.01 mg/kg subcutaneously or intravenously) may increase sinoatrial nodal discharge rate or accelerate AV conduction, but it does not speed up low ventricular pacemakers because the vagus does not innervate the conduction tissue below the bundle of His. Isoproterenol (0.05 to 0.5 μg/kg/min) may increase pacemaker discharge rates and conduction at any level but should be used with care if there are ventricular arrhythmias as well. Slowed conduction through the AV node, especially if induced by digoxin, may be improved by phenytoin. If drug therapy is ineffective or contraindicated, then temporary or permanent artificial cardiac pacing may be needed.
Ectopic Beats and Ectopic Tachycardias
Arrhythmias with premature beats or tachycardias have abnormalities related to increased automaticity of cardiac cells, to increases after depolarization of slow-response fibers, or to uneven conduction times that result in reentry.  Many drugs act in more than 1 way but often have 1 major action, so that it is possible to avoid using 2 drugs with the same basic mode of action.
Many drugs act in more than 1 way but often have 1 major action, so that it is possible to avoid using 2 drugs with the same basic mode of action.
Vagal stimulation (carotid sinus massage, Valsalva maneuver, baroreceptor reflex to hypertension, anticholinesterase drugs) reduces automaticity and shortens the action potential duration and the effective refractory period of atrial muscle; it also slows atrial and atrioventricular (AV) nodal conduction and lengthens the refractory period of the AV node. These actions may abolish paroxysmal supraventricular tachycardia, and they decrease ventricular response in atrial flutter or fibrillation. Because of sparse ventricular innervation, vagal stimulation has little effect or no on ventricular arrhythmias.
Should vagal stimulation fail, the initial drug of choice for treating supraventricular arrhythmias is digoxin. Digoxin may increase ventricular automaticity and thus increases the chances of getting ventricular premature beats and tachycardias. This explains in part why digoxin is not a good choice for treating ventricular arrhythmias. At therapeutic doses, both excitability and conduction velocity are depressed in the atrium. At all doses, AV nodal conduction is slowed so that first the PR interval lengthens; with larger doses, second- or third-degree AV nodal block occurs. Digoxin shortens the action potential duration and the refractory period in atria and ventricles, thus shortening the QT interval. For acute termination of paroxysmal supraventricular tachycardias that rely on the AV node as part of the reentrant circuit, adenosine is the drug of choice.7 Some clinicians use propranolol or verapamil to slow the ventricular response in atrial flutter or fibrillation; verapamil is ineffective in this regard when Wolff-Parkinson-White syndrome is present, as is digoxin, thus both are contraindicated in patients with Wolff-Parkinson-White syndrome8.
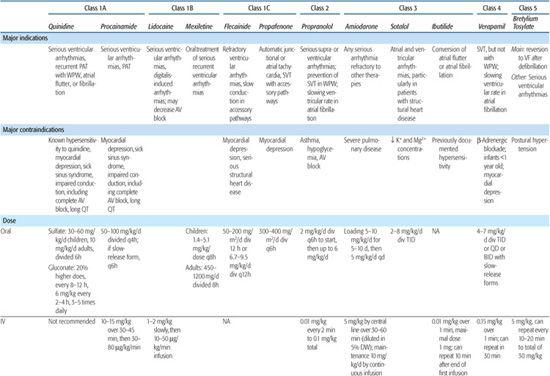
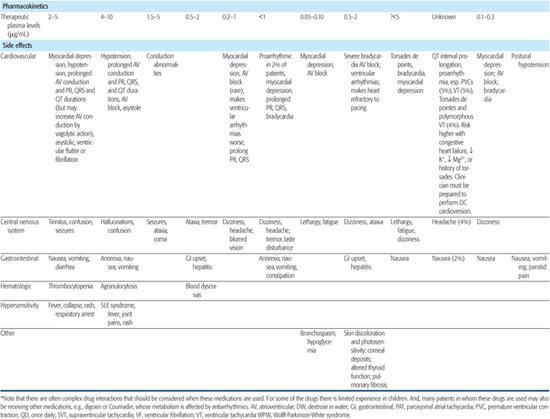
Antiarrhythmic drugs are classified by their major electrophysiological properties; the major groups are described in Table 485-1, as are their main indications and contraindications.
 NONPHARMACOLOGIC MANAGEMENT
NONPHARMACOLOGIC MANAGEMENT
Acute Management
Drug therapy is not always successful in abolishing arrhythmias. Electrical cardioversion by a depolarizing shock is mandatory in ventricular fibrillation and is the method of first or second choice in treating acute tachycardias (paroxysmal atrial or ventricular tachycardias, atrial flutter, or fibrillation), especially if they cause serious cardiovascular difficulties. Rapid overdrive pacing, using temporary electrodes inserted by a transvenous or esophageal route, may be used to drive the heart fast enough to prevent the emergence of ventricular ectopic beats, to abolish reentrant tachycardias,9 or to prevent the recurrence of tachycardias.
Table 485-1. Major Groups of Antiarrhythmic Drugs*
Permanent Antibradycardia Pacing
Permanent pacemaker implantation is often needed to manage bradyarrhythmias. Implantation may be transvenous, by the subclavian or cephalic vein, with the pulse generator placed infraclavicularly. Implantation may, alternatively, be epicardial, with a subxiphoid generator. The epicardial route is reserved for the smallest children and those in whom transvenous pacing is contraindicated because of the risk of thromboembolism (eg, those with an intracardiac shunt and those with no venous access to the ventricle, such as those with the Fontan procedure). 
As a pulse generator nears the end of its battery life (typically 5 to 10 years), it exhibits a characteristic behavior, depending on the manufacturer and model. This is commonly a prolongation of pulse width, slowing of the rate, or the onset of asynchronous pacing. Another common cause of unusual behavior is lead fracture, when the pulse generator may oversense and fail to fire, fail to capture, or revert to asynchronous pacing. All children with implanted pacemakers should have regular follow-up, including transtelephonic monitoring, so that such problems can be detected promptly. 
Implantable Cardioverter-Defibrillator
Children with life-threatening ventricular arrhythmias unresponsive to medical management may need implantation of a device that senses the onset of ventricular fibrillation and delivers 1 or more direct current defibrillation shocks directly to the heart. The lead configuration may be similar to a standard transvenous pacemaker, with a coil in the right ventricular chamber, or the energy may be delivered via epicardial patches or subcutaneous coils.11 The later systems are used in smaller children and in those with single ventricles or a lack of reasonable venous access. These devices have the capability of antibradycardia pacing (in either VVI or DDD configurations), antitachycardia pacing, and cardioversion or defibrillation.
Catheter Ablation
The development of techniques to map and ablate abnormal foci or pathways in the heart using transcatheter radiofrequency energy revolutionized the management of patients with abnormal tachycardia. Currently, ablation may be carried out using either transcatheter radiofrequency energy or via cryoablation (tissue freezing).12 The catheter ablation procedure is performed in the cardiac catheterization laboratory as part of an electrophysiology study. Once the diagnosis is made and the abnormal pathway or focus is located precisely, a special electrode catheter is placed at that site, and ablation energy is delivered. With transcatheter radiofrequency ablation, current passes between the electrode of the catheter and a large chest patch, creating a tiny region of thermal injury on the endocardium. When cryoablation is used, the tip of the catheter is cooled to about −80°C for at least 4 minutes, destroying a small area of endocardial tissue. If properly placed, such lesions may interrupt conduction in an accessory pathway, modify the atrioventricular node, or eliminate an automatic focus. All forms of abnormal tachycardia are amenable to catheter ablation at relatively low risk, although risks are higher in infants. Success rates are over 90% for the common forms of supraventricular tachycardia but are lower for other forms of abnormal tachycardia.13 Radiofrequency ablation has a higher success rate, but cryoablation has a better safety profile, particularly when addressing anatomic substrates that are in close proximity to the atrioventricular node or other critical structures. 
SINUS RHYTHM AND ITS VARIANTS
 SINUS TACHYCARDIA
SINUS TACHYCARDIA
Sinus tachycardia is most often caused by anxiety or exercise but may result from fever, anemia, hypovolemia, shock, congestive heart failure, hyperthyroidism, and many medications. It is often confused with abnormal tachycardias, particularly in sick patients, and differentiation may be difficult. The rate seldom exceeds 200 beats per minute, even in newborns, and varies with time or activity. The electrocardiogram usually shows normal P waves, but at fast rates the P wave may be superimposed on the previous T wave. Vagal stimulation gradually slows the heart rate, in contrast to the abrupt slowing that it may cause with a paroxysmal supraventricular tachycardia. The best diagnostic modality is to treat the likely underlying cause of sinus tachycardia and observe slow resolution.
 SINUS BRADYCARDIA
SINUS BRADYCARDIA
Sinus bradycardia is physiologic in well-trained athletes as well as with vagal stimulation; it is also seen in pathologic states, including increased intracranial pressure, hyperkalemia, hypothermia, hypothalamic seizures, hypothyroidism, hypoxemia, obstructive jaundice, muscarinic poisoning, vagal effects of digitalis intoxication, and surgical damage to the sinoatrial node. It is occasionally seen for no known reason. Note that a slow sinus rhythm implies that the lower pacemakers have an even lower discharge rate than the sinoatrial node.
 SINUS ARRHYTHMIA
SINUS ARRHYTHMIA
Phasic sinus arrhythmia (Fig. 485-5) is a normal variant in which the sinus rate varies; usually, but not always, the rate increases with inspiration and slows with expiration. This arrhythmia usually occurs when there is good vagal tone with a slow or normal heart rate; it is rare in newborns. During expiration the sinus rate may be sufficiently slowed to allow escape beats from an atrial or junctional pacemaker. Sinus arrhythmia with atrial escape beats has also been called wandering atrial pacemaker. The variants of sinus arrhythmia are important only in that they should not be mistaken for serious arrhythmias.
 SINUS ARREST
SINUS ARREST
Sinus arrest is a prolonged cessation of sinoatrial nodal pacemaker activity for more than 2 cycles (eFig. 485.7  ). With no sinus beats, there may be escape beats from a lower pacemaker, but if there are long pauses with no atrial or ventricular activity, then there is failure of the lower pacemakers as well as the sinoatrial node.
). With no sinus beats, there may be escape beats from a lower pacemaker, but if there are long pauses with no atrial or ventricular activity, then there is failure of the lower pacemakers as well as the sinoatrial node.
In sinoatrial block (sinoatrial exit block), the sinoatrial pacemaker is discharging, but occasionally an impulse does not depolarize the atria. This is recognized by pauses that approximate multiples of a normal P-P interval or with Wenckebach-type group beating.
Some children have sick sinus syndrome or bradycardia-tachycardia syndrome. They have episodes of marked sinus slowing or prolonged sinus arrest in which the lower pacemakers do not take over appropriately. As a result, there may be profound slowing of the heart rate or even asystole for several seconds. At other times there may be episodes of tachycardia. This syndrome is seen most often after atrial surgery (eg, repair of atrial septal defects, atrial baffles in transposition of the great arteries, or the atriopulmonary Fontan procedure), but it may rarely arise spontaneously. Attempts to suppress the tachycardias with drugs may lead to unacceptable episodes of slowing or asystole, and permanent implantable pacemakers may be required.
ECTOPIC SUPRAVENTRICULAR RHYTHMS
Because atrial and junctional ectopic pacemakers can produce similar QRS complexes and either similarly abnormal P waves or else no visible P waves, they are usually discussed together.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


