Chapter 46 Vitamin B Complex Deficiency and Excess
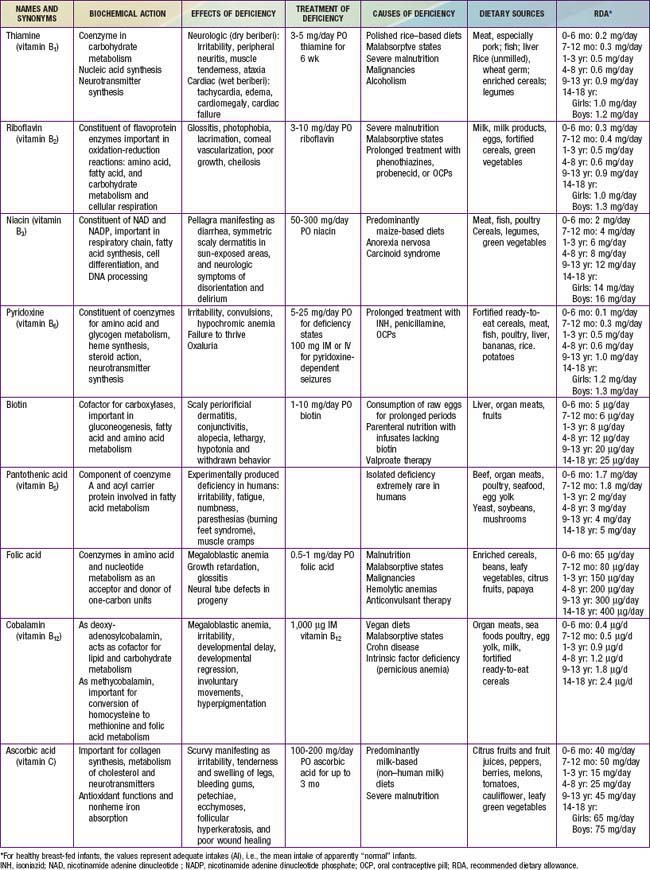
46.1 Thiamine (Vitamin B1)
Boonsiri P, Tangrassameeprasert R, Panthongviriyakul C, Yongvanit P. A preliminary study of thiamine status in northeastern Thai children with acute diarrhea. Southeast Asian J Trop Med Public Health. 2007;38:1120-1125.
Fattal-Valevski A, Kesler A, Sela BA, et al. Outbreak of life-threatening thiamine deficiency in infants in Israel caused by a defective soy-based formula. Pediatrics. 2005;115:e233-e238.
Kornreich L, Bron-Harlev E, Hoffmann C, et al. Thiamine deficiency in infants: MR findings in the brain. Am J Neuroradiol. 2005;26:1668-1674.
Ricketts CJ, Minton JA, Samuel J, et al. Thiamine-responsive megaloblastic anaemia syndrome: long-term follow-up and mutation analysis of seven families. Acta Paediatr. 2006;95:99-104.
46.2 Riboflavin (Vitamin B2)
Deficiency
Clinical Manifestations
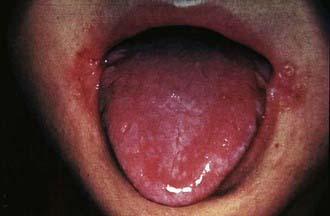
Clinical features of riboflavin deficiency include cheilosis, glossitis, keratitis, conjunctivitis, photophobia, lacrimation, corneal vascularization, and seborrheic dermatitis. Cheilosis begins with pallor at the angles of the mouth and progresses to thinning and maceration of the epithelium, leading to fissures extending radially into the skin (Fig. 46-1). In glossitis, the tongue becomes smooth, with loss of papillary structure (Fig. 46-2). Normochromic, normocytic anemia may also be seen because of the impaired erythropoiesis. A low riboflavin content of the maternal diet has been linked to congenital heart defects, but the evidence is weak.
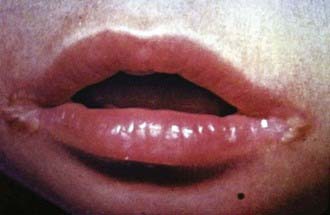
Figure 46-1 Angular cheilosis with ulceration and crusting.
(Courtesy of National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India.)
Prevention
The recommended daily allowance (RDA) of riboflavin for infants, children and adolescents is presented in Table 46-1. Adequate consumption of milk, milk products, and eggs prevents riboflavin deficiency. Fortification of cereal products is helpful for those who follow vegan diets or are consuming inadequate amounts of milk products because of other reasons.