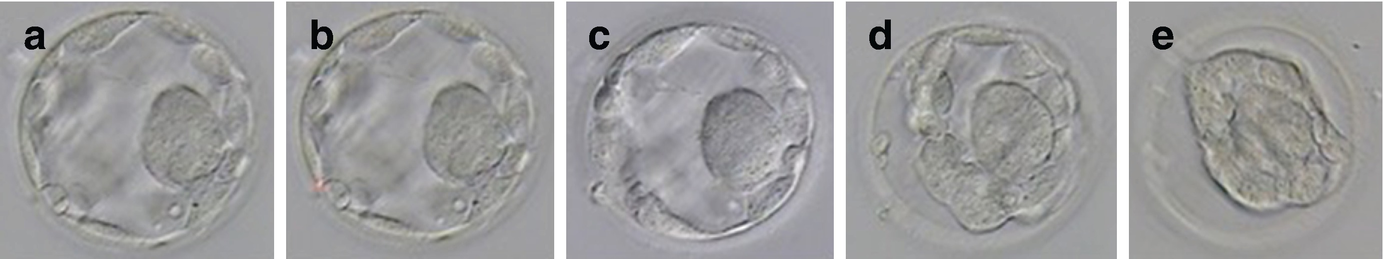
Morphological change of the embryo during vitrifaction: (a) Day 3 embryo before vitrification; (b) dehydration immediately after the exposure to equilibration solution; (c) partial rehydration as cryoprotectants enter the embryo; (d) the embryo regaining original form with cryoprotectant filling; (e) further dehydration in vitrification solution
Artificial collapsing of blastocysts: (a) prior to the artificial shrinkage; (b) a single laser pulse at a junction between two trophectoderm cells away from inner cell mass; (c) beginning of shrinkage 10 s after laser shot; (d) partial shrinkage 30 s after laser shot; (e) blastocyst is fully collapsed and ready to get vitrified
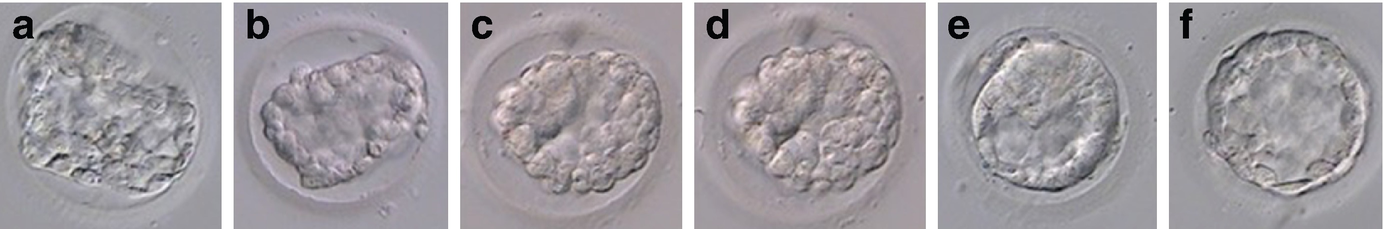
Morphological changes of a human blastocyst during thawing: (a) blastocyst in thawing solution; (b) blastocyst in diluent solution; (c) blastocyst in washing solution 1; (d) blastocyst in washing solution 2; (e) the thawed blastocyst starts to re-expand within 1 h of incubation in culture media; (f) the thawed blastocyst shows 90% blastocoel re-expansion after 2 h of in vitro culture
23.3 Freeze-All Embryos in IVF
The availability of vitrification for embryo storage has also made possible the segmentation of IVF, leading to the so-called freeze-all strategy, which has gained much attention lately [11]. With this strategy, the entire cohort of embryos is cryopreserved following IVF with transfer of a thawed embryo in one or several subsequent cycles [12]. The rationale behind this hypothesis is that the transfer of an embryo into a more “physiologic environment” would result not only in higher pregnancy rates but potentially a decrease in both maternal and perinatal morbidity, when compared with a fresh embryo transfer [13, 14].
23.3.1 Indication of Freeze-All Embryos
The most common reasons for cryopreservation and delayed embryo transfer are the presence of risk factors for ovarian hyperstimulation syndrome (OHSS), the need for pre-implantation genetic diagnosis or testing for aneuploidy (PGD/PGT-A), or the presence of embryo/endometrial asynchrony. The other indications include altered endocrine and cardiovascular profile at the time of transfer (elevated progesterone, hypertension, etc.), inadequate uterine cavity for embryo transfer (i.e., fluid in cavity), and women with poor ovarian response, diminished ovarian reserve (DOR), or advanced reproductive age who seek a strategy to accumulate frozen oocytes or embryos. We still need the evidence to support routine use of the freeze-all policy [15]. However, frozen embryo transfer has become increasingly common and currently there is an accelerating trend toward the implementation for all patient categories [16, 17].
23.3.2 Perinatal and Obstetric Outcomes
First, there is a large body of evidence suggesting that freezing all embryos in a fresh IVF cycle followed by thawed frozen transfer in subsequent cycles (frozen embryo transfer, FET) might improve clinical and ongoing pregnancy rates [14, 18]. A population study suggest that there is a specific risk of blastogenesis birth defects arising very early in pregnancy after IVF or ICSI and that this risk may be lower with use of frozen-thawed embryo transfer [19]. Secondly, compared with embryo transfers following ovarian stimulation, large retrospective cohort studies [20, 21] have shown that frozen-thawed embryo transfers, both at cleavage and blastocyst stages, significantly reduce the rate of ectopic pregnancy. Third, a recent systematic review and meta-analysis suggested that pregnancies occurring after frozen embryo transfer are associated with fewer complications (e.g., lower rates of antepartum hemorrhage) and better neonatal outcomes, including higher birth weight and lower risk of perinatal death [18, 22–24]. These findings have led many to propose that the strategy should apply routinely in IVF. However, these evidences should be taken with caution, since some of the studies available have been criticized due to serious flaws in their design. So more research is still needed to prove the validity of the “freeze-all strategy.”
23.4 Optimal Embryo Transfer Strategy in Diminished Ovarian Reserve Patient
Considering the available evidence that supports freeze-all strategy has great relevance for advances in assisted reproductive technology (ART). Comparisons made between fresh and frozen cycles are mainly based on studies including high responders [15, 18, 25, 26]. Therefore, extrapolating these data to the DOR population should be further investigated.
Several recent studies have explored to analyze the success rate of fresh ET versus FET cycle in cohorts of patients with varying number of oocyte retrieved. Dieamant et al. analyzed the outcomes based on the number of oocytes retrieved and found that the freeze-all strategy appeared beneficial when a high number of oocytes was collected, but that when the mean number of oocytes collected is <15, the freeze-all strategy does not appear to be advantageous [27]. Roque et al. found that when the group with 4–9 oocytes retrieved was analyzed separately, there was no statistically significant difference in pregnancy rates [28]. Recently, in a retrospective study published in 2018, Xue et al. examined 559 poor responders who met Bologna criteria, including 256 in the fresh embryo transfer group and 303 in the freeze-all group [29]. Their result showed similar live birth rate per cycle and per transfer between these two groups. They also found that maternal age at retrieval and number of good quality embryos transferred were significantly associated with the live birth rate. Another retrospective cohort study examined 433 poor responders, including 277 patients who underwent fresh ET and 156 who followed the freeze-all policy. They found the freeze-all strategy, compared with fresh ET, had no impact on IVF outcomes in poor responder patients. Maternal age and number of embryo transferred were the only independent variables associated with ongoing pregnancy rate [30].
Successful implantation of an embryo depends not only on embryo quality but also on endometrial receptivity and the microenvironment for embryo-maternal signaling within the uterine cavity during the peri-implantation period. The main pathophysiologic mechanism involved in the selection of the freeze-all strategy seems to be controlled ovarian stimulation and consequent impairment in endometrial receptivity [31, 32]. We retrospectively analyzed a cohort of 230 cycles in 119 poor ovarian response patients. The IVF cycles were studied in three groups: minimal stimulation cycles, mild stimulation cycles, and conventional high-dose gonadotropin-releasing hormone (GnRH) antagonist cycles. 33 minimal stimulation IVF patients had 41 FET cycles which allowed us to study whether the clomiphene citrate use in minimal stimulation effects were prolonged. We found that endometrial thickness in the minimal stimulation group was significantly lower than the mild and conventional stimulation groups. In patients who underwent minimal stimulation IVF followed by FET, significantly thicker endometrial thickness was achieved during their FET cycles as compared to their minimal stimulation cycles. We concluded that endometrial thickness is impacted during minimal stimulation IVF cycles. Since negative effects on endometrial thickness are not observed in the patients’ subsequent FET cycle, a freeze-all approach is justified to mitigate adverse endometrial effects of clomiphene citrate in minimal stimulation IVF cycles [33].
Moreover, the applicability of vitrification of all embryos to the DOR population can only be a fact when laboratories acquire optimal vitrification systems. However, a consensus is currently lacking in this aspect, and, as a result, ART centers have developed their own freezing strategies based on their personal experiences and choices, which is an important drawback that limits our ability to effectively compare the freeze-all policy available in DOR population [34]. Furthermore, a cost-effectiveness analysis and cumulative pregnancy rates comparison between fresh and frozen embryo transfer is also necessary in order to assess if the potential effects of a freeze-all policy on perinatal outcomes justify the additional cost and extra workload of elective cryopreservation [35].
23.5 Conclusion
Taken together, several observations provide reassuring evidence that the abnormal hormonal milieu and the suboptimal endometrial development observed in conventional ovarian stimulation cycles may be the main risk factor for fresh embryo transfer. The physiological intrauterine conditions of FET may have a positive impact not only on endometrial receptivity but also on early implantation. Although confirmation of the clinical benefits of a freeze-all strategy through well-designed clinical trials is necessary, the freeze-all strategy is an acceptable treatment in DOR patients undergoing minimal and mild stimulation protocols as detailed in this book. In some cases, freezing all embryos might be suggested by physicians as an alternative to cycle cancelation when a fresh transfer would not be advantageous. At the same time, potential costs, delays in treatment, and potential risks associated with this strategy need to be discussed with the patients.

Full access? Get Clinical Tree


