Uterine Ablation Techniques
Frank Tu
INTRODUCTION
There is a long history of attempts to deliver energy to the endometrial lining in order to reduce abnormal menstrual bleeding—dating back to as early as the 1890s. More recently, hysteroscopic resection or hysteroscopic ablation has proven to be of significant value in ensuring the destruction of the endometrium and amelioration of menorrhagia and related symptoms. However, due to concerns about serious complications from fluid overload with conventional hysteroscopic endometrial resection for abnormal uterine bleeding, several global endometrial ablation (GEA) devices were introduced around the beginning of the new millennium. These GEA techniques use radiofrequency, thermal energy, or microwave energy to destroy the endometrium, in order to reduce the amount of cyclical bleeding or, in some cases, even achieve complete amenorrhea.
GEA is indicated for the treatment of dysfunctional uterine bleeding. This can include comorbid leiomyoma for certain devices, but typically a hysteroscopic myomectomy might be a better treatment for a known submucosal leiomyoma. There are several contraindications to consider; these include ongoing pregnancy or desire for future pregnancy, cancer or premalignant change, untreated pelvic inflammatory disease, hydrosalpinx, history of classical cesarean section, or transmural myomectomy. A woman with a cesarean section scar measuring less than 8 to 10 mm on ultrasound should be considered a relative contraindication for GEA or might consider having the procedure done under ultrasound guidance to minimize the risk of perforation. Women with intra-uterine devices (IUDs) in place and, for selected procedures, the presence of intramural leiomyomas and endometrial polyps may benefit from additional pre-ablation procedures.
Benefits of GEA have been supported by multiple head-to-head comparative trials against traditional transcervical endometrial resection, with patient satisfaction ranging from 89% to 98% and amenorrhea rates ranging from 14% to 55%. Patient satisfaction is generally quite high with all the procedures, although it is important to note that one survey has suggested that women are willing to tolerate up to a 50% failure rate with conservative management strategies in order to avoid hysterectomy. Despite the high overall satisfaction with virtually all devices, bipolar ablation has been shown in two head-to-head trials to deliver superior objective results compared to thermal balloon and hydrothermablation, respectively. This may be in part due to differences in how patients are pretreated for these procedures.
The particular attractiveness of GEA over endometrial resection is also evident in the number of cases that increasingly are done in the office or under local anesthetic. That being said, there is still a selective role for endometrial resection in the hands of experienced hysteroscopic surgeons, particularly in patients desiring conservative therapy after failure of initial GEA. Patients do need to be counseled about risks, including the rare risk in women with a prior history of tubal ligation of “postablation tubal pain syndrome,” central hematometra, endometritis with rare reports of sepsis, uterine perforation, and injury to adjacent pelvic
organs. Minor side effects include temporary abdominal cramping in around 10% to 15% of patients treated and a few weeks of vaginal discharge in most patients.
organs. Minor side effects include temporary abdominal cramping in around 10% to 15% of patients treated and a few weeks of vaginal discharge in most patients.
Effective means of contraception are needed if permanent sterilization has not already been assured, as future gestational complications such as uterine dehiscence, intrauterine growth restriction, and preterm delivery have been described in unexpected pregnancies following GEA. Unfortunately, GEA in a subset of women impairs cancer screening due to obliteration of the endometrial cavity, which can obscure the abnormal bleeding that is a hallmark of endometrial cancer.
PREOPERATIVE CONSIDERATIONS
The initial workup of menorrhagia should follow generally accepted clinical practice and includes: (a) excluding pregnancy, (b) completing a pelvic exam and pelvic ultrasonography, (c) obtaining confirmation of a recent negative PAP smear, (d) performing an endometrial biopsy to rule out cervical or endometrial malignancy and pre-malignant changes to the uterus, and (e) potentially performing a hysteroscopy to confirm the appropriateness of the size of the uterus and the absence of intracavitary lesions that might limit the effectiveness of GEA techniques. All methods, except for the Novasure® bipolar electrode array, recommend performing the procedure during the early follicular phase or following a month of hormonal (e.g., progestogen) pretreatment to thin the endometrial lining. Although many patients are comfortable having this done in the ambulatory setting using one of several available analgesic strategies, the clinician must select the best setting based on the examination and his/her assessment of the patient’s tolerance level (Box 1.1).
BOX 1.1
Suggestions for in-office analgesic protocols for GEA procedures
Consider using a combination of the following agents to achieve multimodal pain management. Many practices use all of these in conjunction to achieve optimal comfort. Patient selection is crucial.
Anti-inflammatory: administer 600 to 800 mg of oral ibuprofen every 6 hours beginning in evening prior to procedure, followed by in-office intramuscular injection of ketorolac 30 mg (at least 6 hours after previous NSAID dose)
Muscle relaxant/anxiolytic: oral diazepam 2 to 5 mg 60 minutes prior to procedure
Cervical ripening agent: misoprostol 200 µg at bedtime night prior to procedure—not recommended if doing HTA in office due to concern for spill
Opioid analgesic: 5 to 10 mg of oral hydrocodone or oxycodone, or belladonna and opium rectal suppositories 16.2/30 or 16.2/60, 60 minutes prior to procedure
In-office paracervical block with local anesthetic: must wait 5 to 10 minutes to achieve effect
Patients should consider arranging for transportation to and from procedure if any sedative agents are administered in the perioperative period
To address the issue of a submucosal leiomyoma, or to prevent future pregnancies, some clinicians perform concomitant procedures such as hysteroscopic myomectomy, or tubal sterilization, or IUD placement at the same time as GEA. The Her Option® cryoablation system and MEA® have indications for intracavitary leiomyoma up to 2 and 3 cm, respectively. Published postmarketing experience from Kaiser Permanente suggests that the use of office-based Hydrothermablator® (HTA) can still be effective in the presence of either Type 0 or Type I myomas, although the reported failure rate of 23% at a mean follow-up of ˜ 2.5 years postprocedure was markedly higher than their 3.7% failure rate in myoma-free patients. Similar efficacy has been reported for off-label use of Thermachoice® and Novasure®. There is concern that an immediate prior myomectomy may weaken the uterine tissue and increase the risk of perforation or iatrogenic injury to abdominal organs. Placement of the tubal sterilization implant Essure® must be done after Novasure® ablation due to
the metal content of the implants. While an IUD can be placed after GEA, it may be difficult to remove subsequently due to the resulting fibrosis.
the metal content of the implants. While an IUD can be placed after GEA, it may be difficult to remove subsequently due to the resulting fibrosis.
SURGICAL TECHNIQUE
1. GEA techniques: Patients are placed in slight Trendelenburg position in stirrups and the perineum prepped and draped in the usual fashion. Typically, some sort of IV sedation is given or else office protocols for preoperative pain control are used. The bladder is emptied with a catheter if appropriate. Preoperative antibiotics are usually unnecessary, although patients should be counseled that endometritis can occur rarely after these procedures. A paracervical block is given at 4 and 8 o’clock if indicated and uterus is sounded to confirm appropriateness for each given device. A speculum is placed and the cervix grasped with a tenaculum.
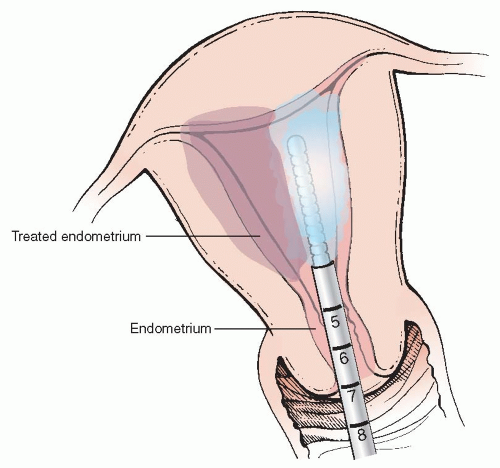
a. Her Option® (Cooper Surgical): The only cryoprobe therapy approach among the five methods reviewed achieves biological effect on the endometrium by cooling the 3.5-cm tip of an intrauterine probe to — 80°C during two freeze cycles of 4 and 6 minutes each, to treat each side of the uterine cavity (Figure 1.1). The procedure is done under ultrasound guidance and usually does not require preoperative cervical dilation as the probe measures 5.5 mm in diameter (and 22 cm in length). Women with uterine cavity lengths between 4 and 10 cm (by sounding) and a uterine volume of 300 ml or less are considered appropriate candidates for treatment with this device.
The active probe is connected to a gas compressor. On activation, a hermetically sealed gas mixture flows into the distal tip of the probe, which permits cooling and heating of the probe. The disposable probe is first tested in the air and confirmed to be able to reach — 50°C in a test freeze. Air is first cleared from the probe channel with a small volume (1-2 ml) of saline and the probe is then inserted under ultrasound guidance toward the right or left fundus. A saline-filled 30-ml syringe is attached to the injection port on the probe, and 5 to 10 ml injected into the cavity to optimize contact of
the probe against the endometrial tissue and enhance sonographic visualization.
the probe against the endometrial tissue and enhance sonographic visualization.
The surgeon begins the freeze cycle by depressing the button on the probe. The formation of the “cryozone” (a discrete dark area detected by ultrasonography at the perimeter of the echolucent cryoprobe) is monitored by ultrasound during the first 4-minute therapy cycle, which should be stopped and the heat button engaged if it reaches closer than 5 mm from the uterine serosal surface. Five to 10 ml of saline is injected into the cavity through the probe after the heat cycle is completed before the probe is withdrawn into the endocervical canal. The probe is then positioned against the opposite cornua and a freeze cycle of up to 6 minutes initiated, again using saline as needed to unseat the probe.
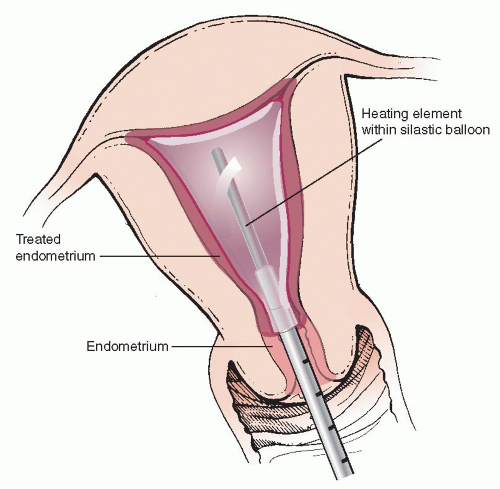
b. Thermachoice® (Ortho Women’s Health): Thermachoice® features a heating element inside a silastic balloon that conforms to the shape of the uterine cavity. The Thermachoice III® has a flexible silastic balloon to optimize fit. The balloon is first checked for leaks out of the package by performing a filling test before placement with a D5W-filled 30-ml luer lock syringe attached to the injection port. Next the balloon is inserted into the endometrial cavity and filled with enough D5W (up to 30 ml) to achieve a stable intrauterine pressure of between 160 and 180 mmHg (Figure 1.2). The heating element is then activated to 87°C for 8 minutes to treat the endometrial cavity. The system will automatically shut off if the intrauterine pressures reach >210 mmHg or <45 mmHg (such as with sudden loss of balloon integrity or uterine perforation) during a heating cycle; or balloon temperatures exceed >95°C for more than 2 seconds or <75°C for 15 seconds. Original approval by the FDA was for uterine sizes between 4 and 12 cm with normal cavity contour.
c. Hydrothermablator® (HTA, Boston Scientific):




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree