Upper limb reconstruction in persons with tetraplegia
Nonoperative management
Indications
Contraindications
All persons with tetraplegia
None, except for a really sick patient who is unable to tolerate any treatment
Contracture prevention
Joint mobilization
Patient and caregiver education
Blood pressure contracture
Decubiti prevention
Psychology support
Upper limb reconstruction in persons with tetraplegia: physical/occupational therapy recommendations |
|---|
Maintain supple range of motion |
Prevent or alleviate contracture |
Mobilize the patient slowly with awareness of potential hypotension and hypertension issues |
Minimize possibility of decubiti in bed and in wheelchair |
Ascertain appropriate wheelchair seating and propulsion process |
High-Level Tetraplegia
High-level tetraplegia results in many particular impediments to improve upper limb function. The main obstructions involve the dependence upon the respirator and the shoulder girdle. The phrenic nerve arises from C3, C4, and C5 that keep the diaphragm alive. The loss of diaphragmatic function is devastating as the person is totally dependent upon the respirator 24–7. Any malfunction or mucous plug is a dire emergency, and imminent death is a real possibility. In fact, the life span in persons with high-level tetraplegia is roughly a decade, and pulmonary complications contribute to their shortened life span. There has been a variety of surgical procedures to negate respiratory dependency. The two options are pacing the phrenic nerve or nerve transfer to the phrenic nerve (Yang et al. 2011; Tibballs 1991). Phrenic nerve pacing involves a pacemaker that stimulates the nerve via a nerve cuff (Fig. 1). If the diaphragm does not respond to stimulation, a nerve transfer may be performed. The transfer can be an active motor nerve, such as the spinal accessory nerve. In addition, the transfer can be a donor nerve that can be stimulated, such as the intercostal nerve(s) below the level of injury (Krieger and Krieger 2000; Tibballs 1991) (Fig. 2). This surgery is relatively new and innovative; however, the benefits can be life changing with lessened ventilator dependence.
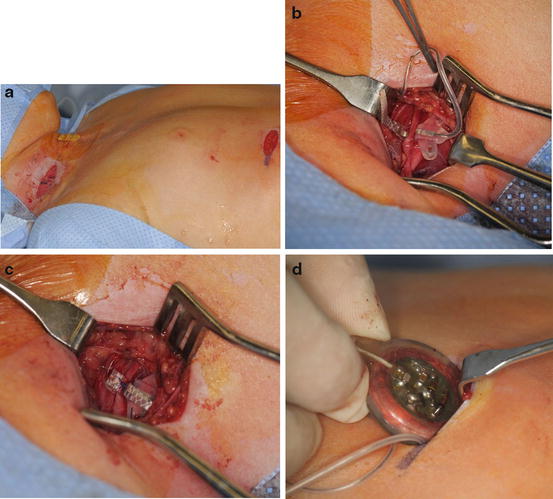
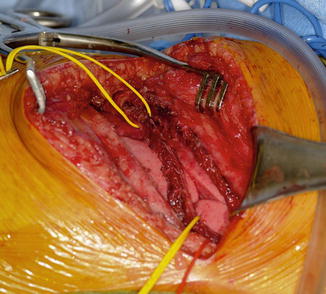

Fig. 1
A 6-year-old girl with a high-level tetraplegia, ventilator dependency, and no function below the neck. Electrodiagnostic studies found the phrenic nerve responsive to stimulation and she was deemed a candidate for phrenic nerve pacing (Courtesy of Shriners Hospital for Children, Philadelphia). (a) Patient positioned supine on operating table. Neck incision is for phrenic nerve isolation and abdominal incision is for pacemaker insertion. (b) Isolation of phrenic nerve isolation and electrode wrapped around nerve. (c) Electrode secured around phrenic nerve. (d) Pacemaker placed in abdominal pocket

Fig. 2
A 7-year-old girl with high-level tetraplegia and ventilator dependency. Left thoracotomy performed for exposure of phrenic nerve and harvest of intercostal nerve (Courtesy of Shriners Hospital for Children, Philadelphia)
Technique: Intercostal-to-Phrenic Nerve Transfer
Intercostal-to-phrenic nerve transfer |
|---|
Preoperative planning |
OR table: regular |
Position/positioning aids: lateral decubitus, meticulous padding of all bony prominences, thoracic surgeon |
Fluoroscopy location: none |
Equipment: standard, chest tube, phrenic nerve stimulator |
Tourniquet: none |
The patient is placed in lateral decubitus position with the affected side up. A posterolateral thoracotomy is performed at the 5–6 interspace (Fig. 2). The lung is retracted to expose the phrenic nerve, which is identified and stimulated to confirm diaphragmatic paralysis. The phrenic nerve is transected 5 cm proximal to its insertion into the diaphragm (Fig. 3). The intercostal nerve from the 5–6 interspace is identified from the posterior axillary fold to the costochondral junction (Fig. 4). The nerve is cut at the costochondral junction (Fig. 5). A 5 mm cuff of surrounding muscular tissue is preserved around the nerve to maintain its vascularity. The intercostal nerve is directly repaired to the phrenic nerve without tension (Fig. 6). The coaptation is performed with sutures and/or fibrin glue.
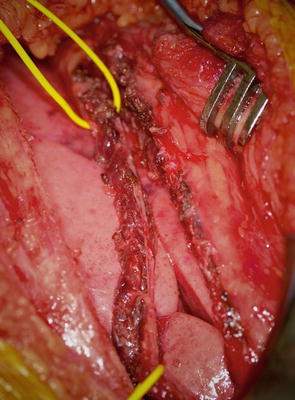
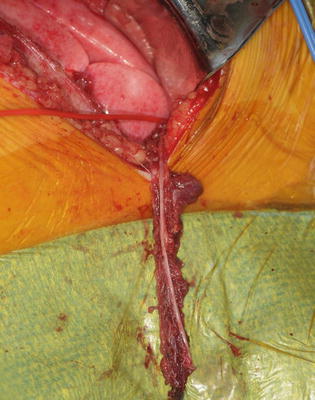
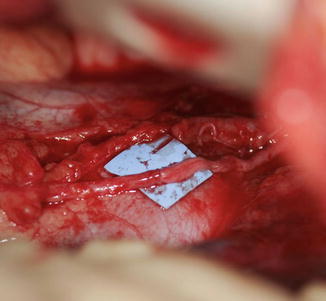

Fig. 3
Phrenic nerve isolated and transected 5 cm proximal to diaphragm (Courtesy of Shriners Hospital for Children, Philadelphia)

Fig. 4
Intercostal nerve isolated from costochondral junction to posterior axially fold with cuff of muscle (Courtesy of Shriners Hospital for Children, Philadelphia)

Fig. 5
Intercostal nerve cut at costochondral junction (Courtesy of Shriners Hospital for Children, Philadelphia)

Fig. 6
Intercostal and phrenic nerve repaired end to end with fibrin glue (Courtesy of Shriners Hospital for Children, Philadelphia)
The phrenic pacer electrode is then placed around the phrenic nerve 1 cm distal to the coaptation site (Fig. 7). The pacer line is tunneled to the pacemaker’s receiver, which is implanted on the anterior aspect of the chest wall or abdomen. A chest tube is inserted followed by standard closure.
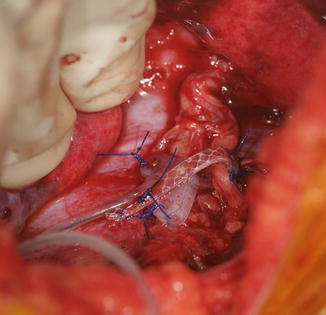

Fig. 7
Stimulator electrode wrapped around the phrenic nerve (Courtesy of Shriners Hospital for Children, Philadelphia)
Following the surgery, the patient is admitted to the intensive care unit for routine postoperative care. The pacemaker is activated 3 months after surgery, and the diaphragm is assessed for responsiveness. If the diaphragm is unresponsive, the patient is returned to mechanical ventilation and the diaphragm is reassessed at weekly intervals. Once the diaphragm is found to be reactive, a pacing schedule is instituted. The duration of pacing is increased as the parameters improve (tidal volume, end tidal CO2, and patient comfort).
Intercostal-to-phrenic nerve transfer |
|---|
Surgical steps |
Posterolateral thoracotomy is performed at the 5–6 interspace |
Phrenic nerve identified and traced toward the diaphragm |
Phrenic nerve transected 5 cm proximal to its insertion into the diaphragm |
Intercostal nerve with cuff of muscle isolated from costochondral junction to posterior axillary fold |
Intercostal nerve cut at the costochondral junction and repaired end to end to the phrenic nerve |
Phrenic pacer electrode is then placed around the phrenic nerve 1 cm distal to the repair site |
Pacer line is tunneled to the pacemaker’s receiver, which is implanted on the anterior aspect of the chest wall or abdomen |
Chest tube and closure |
Intercostal-to-phrenic nerve transfer |
|---|
Postoperative protocol |
Intensive care unit for routine postoperative care |
Pacemaker is activated 3 months after surgery and the diaphragm is assessed for responsiveness |
Weekly assessments of the diaphragm’s responsiveness |
Once the diaphragm is found to be reactive, a pacing schedule is instituted |
Duration of pacing increased as parameters improve (tidal volume, end tidal CO2, and patient comfort) |
Shoulder Girdle
The shoulder girdle instability plagues those persons with high-level tetraplegia. The scapulothoracic and glenohumeral joints lack stability and motion. The loss of the parascapular stabilizers including the rhomboids leads to fragrant instability of the scapulothoracic joint that is particularly problematic (Fig. 8). Decubiti can develop between the scapula and wheelchair. In addition, the total loss of proximal stability negates any ability to position the hand in space. Attempts to minimize the scapulothoracic joint instability have resulted in mediocre results and a fairly high complication rate (Pahys et al. 2009).
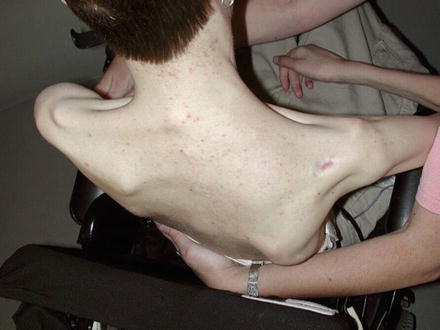

Fig. 8
High-level tetraplegia with profound instability of the scapulothoracic joint (Courtesy of Shriners Hospital for Children, Philadelphia)
The unstable glenohumeral joint is yet another obstacle for persons with high-level tetraplegia. The lack of rotator cuff musculature and deltoid function results in instability of the glenohumeral joint with subluxation and possible pain. The surgical options to restore stability are limited. The absence of parascapular stabilizers negates the possibility of shoulder arthrodesis as this procedure would result in further scapulothoracic instability. In addition, the only available donor muscle for transfer is the trapezius and levator scapula. In this scenario, the trapezius is transferred to the humeral head and the levator scapula to the supraspinatus. This procedure will lessen the humeral head subluxation but result in minimal active shoulder motion. The best treatment algorithm for this difficult problem remains unsolved.
Functional Electrical Stimulation
Functional electrical stimulation (FES) for persons with tetraplegia was here and gone. The system provided substantial improvements for those adults and children with high-level tetraplegia. FES involved coordinated stimulation of innervated muscles below the level of injury to result in prehension. Lateral pinch and grasp were attainable goals that resulted in an increase in function and less dependence on caregivers (Kilgore et al. 1997).
For a variety of reasons, FES became unavailable despite the initial promising reports. There are substantiated rumors that a newer system will be released with improved technology and more versatility than the older system. Many persons with high-level tetraplegia anxiously await the release of such a device to improve function and enhance independence in daily life.
Incomplete Tetraplegia
There are a variety of etiologies for incomplete tetraplegia including trauma, transverse myelitis, infection, and tumors. Incomplete tetraplegia typically results in an asymmetric lesion and differences in arm usage. The discrepancy in upper limb usage and the frequent preservation of some lower limb function confound the treatment algorithm. A meticulous upper extremity assessment coupled with time spent with an intuitive therapist is helpful when formulating a surgical plan. The surgical goals in complete and incomplete are similar with an emphasis on restoring upper limb function and increasing spontaneity and independence.
Mid-level Cervical Tetraplegia
The most common level of cervical spinal cord injury is C5–C6. The preservation or recovery of C5 and C6 nerve roots offers opportunity for upper limb function. Nerve roots C5 and C6 motor the shoulder girdle, elbow flexion, forearm supination, and some wrist extension. These motions stabilize the shoulder girdle and provide a foundation for upper limb reconstruction. Lower cervical spinal cord injury preserves additional muscles and offers more reconstructive options.
The upper extremity classification of tetraplegia combined with the “hierarchy of hand function” or “reconstructive ladder” of hand function yields a relatively straightforward algorithm for reconstruction.
Classification
The classification for upper extremity impairment and surgical management has been well established (Kozin 2007; Zlotolow 2011). The goal of the international classification for surgery of the hand in tetraplegia (ICSHT) is to guide management (Table 1). The objective is to identify those muscles distal to the elbow that are potentially transferrable. The classification follows the American Society for Spinal Cord Injury cataloging but emphasizes muscle strength. Therefore, each muscle below the elbow that is a grade 4 or has greater strength adds additional gradation. For example, if the brachioradialis is present and strong, but no other muscles are of similar strength, the ICSHT is grade I. The subsequent muscle strength and gradation are based upon primary nerve innervation. In other words, the extensor carpi radialis longus is mainly a C6 muscle. The extensor carpi radialis brevis is principally a C7 muscle as are the pronator teres, flexor carpi radialis, extensor digitorum communis, extensor pollicis longus, and flexor digitorum superficialis. The flexor digitorum profundus and intrinsic muscles are principally innervated by C8 and T1 nerve roots. The addition of another strong muscle adds a grade to the classification.
Table 1
International classification for surgery of the hand in tetraplegia (ICSHT)
Grade | Muscle below the elbow function | Motor description |
|---|---|---|
0 | None | Elbow flexion and forearm supination |
1 | Brachioradialis | Forearm stabilization with some supination/pronation |
2 | Extensor carpi radialis longus | Wrist extension |
3 | Extensor carpi radialis brevis | Strong wrist extension |
4 | Pronator teres | Forearm pronation |
5 | Flexor carpi radialis | Wrist flexion |
6 | Extensor digitorum communis | Finger extension |
7 | Extensor pollicis longus | Thumb extension |
8 | Flexor digitorum superficialis | Incomplete finger flexion |
9 | Flexor digitorum profundus | Complete digital roll-up |
X | Exceptions | Variable |
Treatment
Elbow
Persons with C5–C6 spinal cord injury lack elbow extension. The lack of elbow extension hampers function. Deficient elbow extension has multiple negative ramifications. Workable reach space is dramatically decreased as the arm is unable to reach out into space. Daily activities for persons with spinal cord injury are compromised such as pushing a wheelchair, transferring in and out of a bed or a chair, and weight shifting for decubitus prevention. Restoration of elbow extension can have a dramatic increase in reachable workspace, facilitate wheelchair propulsion (even popping wheelies), and enable transfers in and out of bed or chair. In addition, weight shifts are easier, which lessen the chances of decubiti development.
Recent investigation into nerve transfers has generated promise; however, the current gold standard is tendon transfer (Bertelli and Ghizoni 2013). The two main donors are the biceps and the posterior deltoid muscles (Mulcahey et al. 2003; LeClerq et al. 2008). Each procedure has its own nuances, positives, and negatives. The biceps-to-triceps transfer is easier and has a less rigid postoperative rehabilitation. The posterior deltoid transfer requires an intervening graft that complicates the technique and adds greater postoperative restrictions.
Technique: Biceps-to-Triceps Transfer
Operative Prerequisites
Active brachialis and supinator muscles are requirements to biceps transfer to maintain elbow flexion and forearm supination (Kozin 2003; Kuz et al. 1999). The evaluation of their presence requires a vigilant physical examination of elbow flexion and forearm supination strength. The brachialis and supinator muscles can be assessed independent of the biceps muscle. Effortless forearm supination without resistance will cause supinator function that is palpable along the proximal radius. Likewise, powerless elbow flexion causes palpable brachialis contraction along the anterior humerus deep to the biceps muscle. Equivocal cases require supplementary evaluation to guarantee adequate supinator and brachialis muscle activity. Injection of the biceps muscle with a local anesthetic (e.g., bupivacaine) induces temporary paralysis. Subsequently, the examiner can assess independent assessment of brachialis and supinator function via elbow flexion and forearm supination, respectively.
A supple elbow with near-complete range of motion is also necessary. Patients with an elbow contracture require resolution of the contracture prior to surgery. Therapy and/or serial casting can lessen the contracture. Surgery is deferred until the contracture is less than 20°. A greater contracture negates the biceps tendon from reaching the olecranon at surgery.
Biceps-to-triceps transfer |
|---|
Preoperative planning |
OR table: regular |
Position/positioning aids: supine, meticulous padding of all bony prominences |
Fluoroscopy location: none |
Equipment: standard |
Tourniquet: sterile |
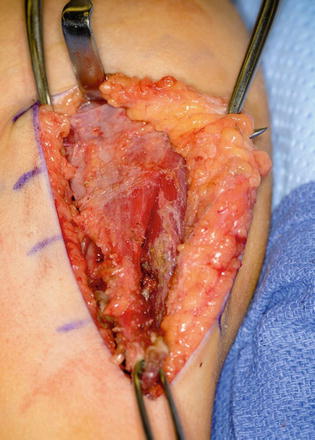
The patient is placed supine on the operating room table. The entire extremity is prepped and draped. A sterile circular tourniquet is applied (HemaClear, OHK Medical Devices, Grandville, Michigan, USA) that exsanguinates during application. An S-shaped incision is made along the medial arm, across the antecubital fossa, and over the brachioradialis muscle belly. Skin flaps are elevated and the biceps tendon is traced to its insertion into the radial tuberosity. There are large crossing veins that require ligation. The lacertus fibrosis can be incorporated into the transfer or left behind (Kozin 2003; Kuz et al. 1999; Revol et al. 1999; Kozin et al. 2010). The tendon is released directly from the radial tuberosity to maximize length. The tendon and muscle belly are mobilized proximal into the arm to maximize excursion and to improve line of pull (Fig. 9). The musculocutaneous and lateral antebrachial nerves must be protected.
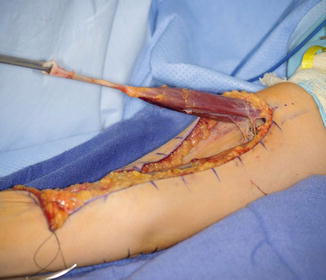

Fig. 9
Eighteen-year-old female with spinal cord injury and lacks elbow extension necessitating biceps-to-triceps transfer. Biceps harvested from radial tuberosity and proximal dissection performed to improve excursion (Courtesy of Shriners Hospital for Children, Philadelphia)
Only the medial side of the arm, median nerve, brachial artery, and ulnar nerves is isolated. A posterior incision is made around the olecranon and extended in a proximal direction along the triceps tendon. A subcutaneous tunnel is created between the medial brachium and posterior incision for tendon passage (Fig. 10). The tendon was passed over the median ulnar nerves, however; the advances in neuroregeneration has changed our practice. The tendons are passed over the median nerve but under the ulnar nerve to avoid any ulnar nerve iatrogenic compression (Fig. 11). A Krackow suture is placed in the tendon with the two suture ends left long.
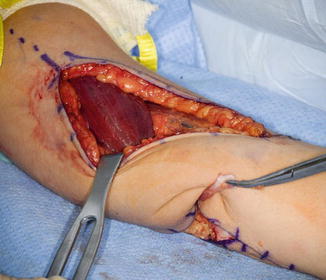
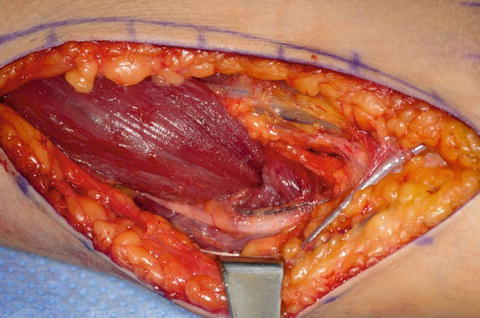

Fig. 10
Biceps passed along the medial side of the arm to the posterior incision beneath the ulnar nerve (Courtesy of Shriners Hospital for Children, Philadelphia)

Fig. 11
Biceps passed beneath the ulnar nerve (Courtesy of Shriners Hospital for Children, Philadelphia)
The triceps tendon is split and the tip of the olecranon is exposed. A large-bore blind bone tunnel is made in the olecranon for acceptance of the biceps tendon. The tendon is passed through the medial leaflet of the triceps tendon in preparation of docking into the bone tunnel (Fig. 12). The tendon is docked using two posterior unicortical holes and suture retrievers (suture retriever, Smith and Nephew, Andover, Massachusetts, USA) (Kozin and Zlotolow 2012) (Fig. 13). The elbow is place in full extension and the two ends are drawn into the bone tunnel using suture retrievers (Fig. 14). The sutures are tied over the posterior olecranon cortex. Additional suturing is performed during closure of the triceps split with incorporation of the biceps tendon. Following closure, a well-padded long arm cast is applied with the elbow in extension. The wrist is included within the cast and the hand position depends upon concomitant procedures performed for hand function.
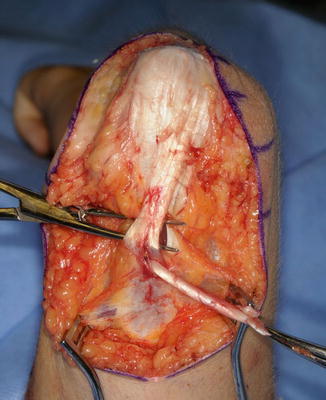
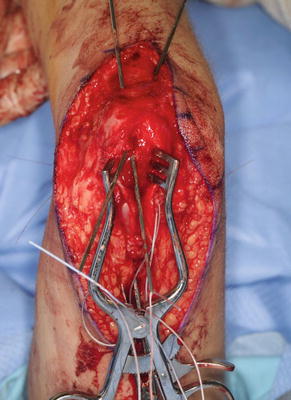
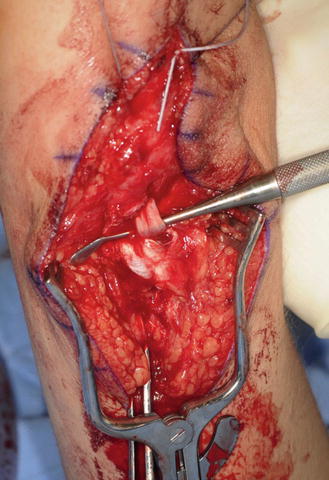

Fig. 12
Biceps tendon passed through triceps tendon prior to docking into olecranon (Courtesy of Shriners Hospital for Children, Philadelphia)

Fig. 13
Suture retrievers to facilitate docking biceps tendon into osseous tunnel (Courtesy of Shriners Hospital for Children, Philadelphia)

Fig. 14
Biceps tendon docked into olecranon (Courtesy of Shriners Hospital for Children, Philadelphia)
Biceps-to-triceps transfer |
|---|
Surgical steps |
S-shaped incision is made along the medial arm, across the antecubital fossa, and over the brachioradialis muscle belly |
Biceps tendon traced to insertion into the radial tuberosity, released, and mobilized in a proximal direction |
Posterior incision around the olecranon and extended in a proximal direction, triceps tendon is split and the tip of the olecranon is exposed |
Biceps tendon passes from anterior to posterior via spacious tunnel that runs over the median nerve and deep to the ulnar nerve |
Biceps tendon passes through the triceps leaflet and secured within the osseous tunnel using a docking technique |
Biceps-to-triceps transfer |
|---|
Postoperative protocol |
Elbow extension cast for 1 week |
Gradually mobilize elbow and initiate transfer training at 15° per week, using a dial-hinge brace Bledsoe brace (Bledsoe Brace Systems, Grand Prairie, Texas, USA) |
Tendon transfer firing and reeducation instituted |
Protective splinting of the elbow is continued until 3 months after surgery |
Biceps-to-triceps transfer | |
|---|---|
Potential pitfalls and preventions | |
Potential pitfall | Pearls for prevention |
Pitfall #1 Bleeding during biceps harvest | Large crossing veins require careful ligation during dissection |
Pitfall #2 Inadequate biceps length to reach olecranon | Resolve any elbow contracture prior to surgery |
Pitfall #3 Difficulty firing transfer after surgery | Try alternative methods, such as biofeedback |
Pitfall #4 Difficulty regaining elbow flexion | Be patient, avoid passive elbow flexion until 3 months following surgery |
Technique: Posterior Deltoid Transfer
Operative Prerequisites
The posterior deltoid must have adequate strength for motor elbow extension. Shoulder extension and palpation best assesses posterior deltoid strength and turgor. The examination can be performed with the patient seated, although placing the patient prone provides a more reliable assessment. Similar to biceps-to-triceps transfer, a supple elbow with near-complete range of motion is also necessary. Patients with an elbow contracture require resolution of the contracture prior to surgery.
Posterior deltoid transfer |
|---|
Preoperative planning |
OR table: regular |
Position/positioning aids: supine with bump under shoulder, meticulous padding of all bony prominences. Can also position in lateral decubitus position if performing isolated posterior deltoid transfer |
Fluoroscopy location: none |
Equipment: standard, allograft |
Tourniquet: none |
The patient is positioned in the lateral decubitus position with the affected side up (LeClerq et al. 2008; Mulcahey et al. 2003). An axillary roll is applied. All bony prominences are padded. Prior to prepping and draping, Marcaine with epinephrine is instilled in the planned incision sites. The upper extremity and hemithorax are then prepped and draped in usual sterile fashion. A gently curved incision is made over the deltoid muscle (Fig. 15). Skin flaps are elevated. The axillary nerve is identified entering the deltoid muscle (Fig. 16). The posterior half of the deltoid is selected for transfer (Fig. 17). A periosteal sleeve is elevated with the posterior deltoid. Proximal dissection is performed to improve the excursion and line of pull.
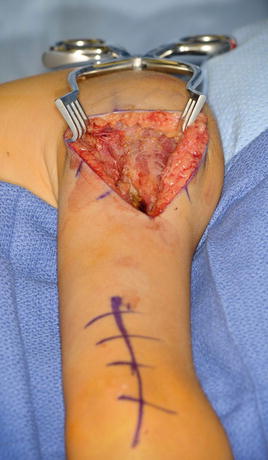
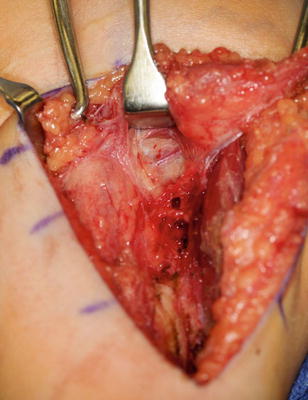

Fig. 15
Incisions for deltoid-to-triceps transfer (Courtesy of Shriners Hospital for Children, Philadelphia)

Fig. 16
Axillary nerve delineated and protected (Courtesy of Shriners Hospital for Children, Philadelphia)