Photograph of placental fronds demonstrating an adequate sample volume and quality for analysis.
For transcervical CVS, the cervix is visualized with a Cuscoe’s speculum. The cervix is cannulated under continuous ultrasound guidance. Techniques for obtaining the sample depend on operator experience, but there is some evidence to suggest an advantage of small biopsy forceps over aspiration cannulae.
Amniocentesis
Local anesthetic was shown to have no effect on pain scores for amniocentesis, and is used by just 4% of specialist centers in the UK[8]. Where possible, the needle path is transamniotic and the placenta is avoided. However, if transplacental passage of the needle provides the only safe access to an adequate pool of liquor, there is no evidence of increased procedure-related complications[2]. The placental cord insertion should be avoided. A 20–22G needle should be inserted under continuous ultrasound control. The stylet of the needle can be removed when the tip is clearly into the amniotic sac, and aspiration attempted. Occasionally the needle will be visible in the sac with ultrasound but aspiration is not possible as the needle is obstructed by amniotic membrane. When this occurs the needle can be advanced slightly or rotated to free the tip. A 15–20 mL sample is generally adequate for analysis and should be transported in a plastic container. When sufficient volume has been aspirated, the stylet of the needle is reinserted and the needle removed with continuous ultrasound guidance. The color of the sample and presence of any bloodstaining should be recorded.
Fetal blood sampling
Local anaesthetic is advisable for this procedure. In some cases, particularly where FBS is combined with transfusion, fetal paralysis usually using vecuronium will need to be considered. A 20G needle is inserted at a preselected site to approach the target using continuous ultrasound control. When approaching cord vessels, the needle should be placed in light contact with the intended portion of cord, and then advanced swiftly into the target vessel. This assists with piercing the Wharton’s Jelly and avoids glancing the needle off the target (Figure 27.2). The intrahepatic portion of the umbilical vein can be approached through the fetal liver or along the entry of the umbilical cord. After entering the vessel, the stylet of the needle can be removed and a 1-mL syringe aspirated to confirm intravascular placement. A 1–2 mL sample of fetal blood will be adequate for hemoglobin, fetal virology or karyotype. Blood should be aspirated into a lithium heparin tube (EDTA is used for microarray). A rapid estimate for fetal hemoglobin can be obtained with a point-of-care test, and confirmed with a full blood count (Figure 27.2).
After removing an adequate volume, the stylet of the needle is replaced and the needle removed under ultrasound guidance. It is normal to see a small amount of turbulence in the amniotic fluid surrounding the insertion site or a small echogenic area as fetal blood seals the puncture.
Performing the procedure
Preparation
The tray for invasive prenatal tests should be set-up in a uniform way for each procedure, and asepsis of the tray maintained throughout (Figures 27.3 and 27.4).
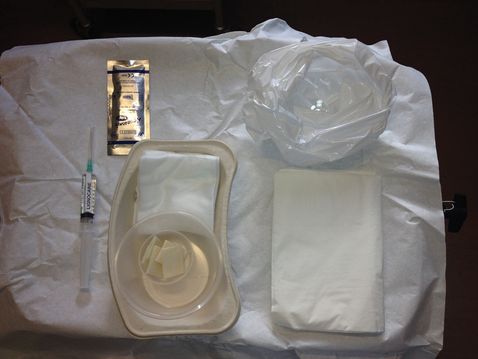
Standard tray for ultrasound-guided diagnostic procedures.
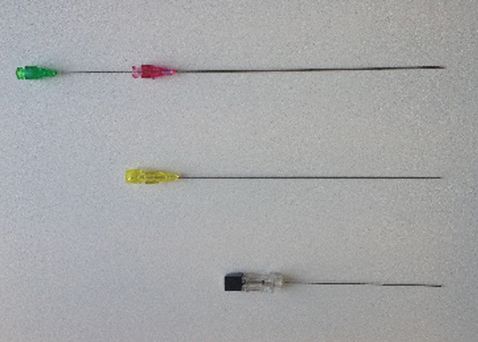
Needles for chorionic villus sampling (green/purple), fetal blood sampling (yellow) and amniocentesis (black).
When preparing for FBS, possible actions in case of persistent fetal bradycardia should be discussed with the couple. When the fetus is considered viable, arrangements should be made with the delivery suite for the possibility of a category 1 cesarean section before beginning the procedure.
A trained chaperone who can assist in supporting the patient and receiving the sample should be available. The patient should be offered the choice to be accompanied by her partner or other support person during the procedure, who can be seated at her side clear of the intended aseptic field.
The ultrasound probe should be enclosed in a sterile bag, and a separate sterile gel should be available. Use a two-dimensional ultrasound probe and select a setting for invasive procedures with a wide-angled field to assist tracking of the needle. The abdominal skin should be cleansed using disposable chlorhexidine wipes, and sterile disposable drapes applied.
Prior to commencing the procedure, an assessment should be made of the most suitable approach to the target. For amniocentesis, this will include avoiding transplacental passage of the needle where possible. The patient should be positioned supine; it may also be helpful to tilt the patient to access the target with the needle, in which case the patient should be stabilized and made comfortable by adjusting the bed or using pillows or a wedge. Contrary to common belief, a full bladder is rarely needed for invasive procedures. More often than not it is simply a hindrance that makes women more uncomfortable. For transcervical CVS, a full bladder is required, a full bladder is also required in some cases with a retroverted uterus for transabdominal CVS. FBS is possible but more technically demanding prior to 20 weeks’ gestation due to smaller targets and more friable tissues. When selecting the intended targets, fixed, stable areas, such as the placental cord insertion or intrahepatic portion of the umbilical vein, are more favorable, whereas free cord loops are more inclined to move and tear. A contingency plan should be made for preferred and approachable targets in each case.
When preparing and carrying out transabdominal procedures, the two-dimensional transabdominal probe should be utilized at 90° to the floor and the uterus viewed in transverse section. The needle should be introduced in-line with the probe, and the probe slightly adjusted to keep the full length of the needle in view throughout. For all procedures, lateral movement of the needle should be avoided.
For any procedure, a more experienced operator should be consulted if two attempts at uterine insertion have failed to produce an adequate sample for analysis[2]. Where difficulties can be anticipated (obesity, multiple pregnancy, poor access to placental cord insertion), these should be prepared for in setting up the procedure, including considering support from a second experienced operator (Figures 27.5 and 27.6).
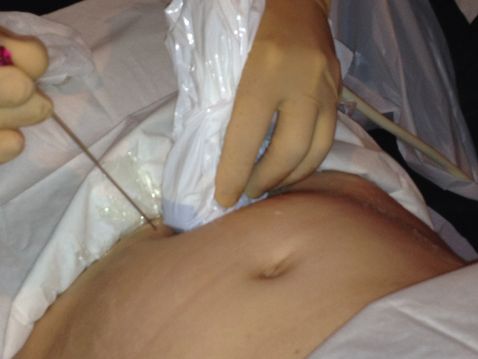
Correct alignment of two-dimensional ultrasound probe and needle.
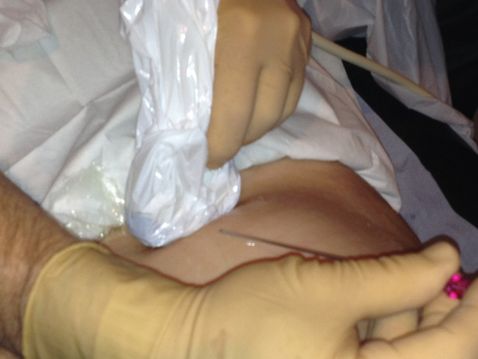
Incorrect alignment of the two-dimensional ultrasound probe and needle.
Postprocedure management
At completion of the procedure, the fetal heartbeat should be demonstrated to the woman. The needle insertion site should be cleaned and covered with a small sterile dressing, and anti-D arranged for Rhesus (Rh) negative women (250 units IM <20 weeks’ gestation, 500 units >20 weeks), according to RCOG guidance[9]. Time should be allowed to ask questions following the procedure. The procedure should be documented in a standardized format stating indication, type of procedure, needle gauge, entries, complications and provision of anti-D where applicable.
Women should be advised to expect mild discomfort at the needle insertion site and mild period-type abdominal discomfort, during the first 24–48 h for which paracetamol should be sufficient. They should be advised to avoid strenuous activity for 48 h, and activities involving bending, stretching or lifting. Many women will prefer to be at home during this time. It should be explained that this is for her comfort, but does not prevent procedure-related pregnancy loss occurring.
If more severe abdominal pain occurs, vaginal loss or bleeding, or the woman feels systemically unwell, she should seek advice from the hospital.
A rapid karyotype from quantitative fluorescent PCR (QF-PCR) result is usually available within 4 working days, microarray within 10–14 days and full culture within 21 days. The method for communicating results should also be discussed and agreed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


