Treatment
Hyperbilirubinemia can be treated in three ways: (a) exchange transfusion removes bilirubin mechanically; (b) phototherapy converts bilirubin to products that can bypass the liver’s conjugating system and be excreted in the bile or in the urine without further metabolism; and (c) pharmacologic agents that interfere with heme degradation and bilirubin production, accelerate the normal metabolic pathways for bilirubin clearance, or inhibit the enterohepatic circulation of bilirubin. Phototherapy is the most common treatment in use for hyperbilirubinemia; exchange transfusions generally are reserved for phototherapy failures. The bilirubin level at which intervention is necessary is still a contentious issue.
TABLE 35-30 GUIDELINES FOR USE OF PHOTOTHERAPY AND EXCHANGE TRANSFUSION IN PRETERM INFANTS BASED ON GESTATIONAL AGE | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Principles Underlying the Recommendations
Recommendations and guidelines for the use of phototherapy and exchange transfusion in term, near-term and preterm infants are provided in Tables 35-29 to 35-32 and Figs. 35-22 and 35-23. It would be ideal if the guidelines for the implementation of phototherapy and exchange transfusion relied on evidence-based estimates of when the benefit of these interventions exceeded their risks and costs (76). Ideally, these estimates should come from randomized trials or high quality, systematic observational studies but such studies are rare (139,140). Thus, treatment guidelines must rely on relatively uncertain estimates of risks and benefits
and the recognition that using a single TSB level to predict long-term behavioral and developmental outcomes is not reliable and will lead to conflicting results (139,140).
and the recognition that using a single TSB level to predict long-term behavioral and developmental outcomes is not reliable and will lead to conflicting results (139,140).
TABLE 35-31 GUIDELINES ACCORDING TO BIRTH WEIGHT FOR EXCHANGE TRANSFUSION IN LOW-BIRTH-WEIGHT INFANTS BASED ON TOTAL BILIRUBIN (mg/dL) AND BILIRUBIN-TO-ALBUMIN RATIO (mg/g) (WHICHEVER COMES FIRST) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
The background to treatment decisions for hyperbilirubinemia has been provided (see section on Bilirubin Toxicity.) The basic principles underlining the recommendations given in Tables 35-29, 35-30, 35-31 and 35-32 and Figs. 35-22 and 35-23 are as follows:
Phototherapy is a highly effective method for preventing and treating hyperbilirubinemia (see Phototherapy below). The main demonstrated value of phototherapy is that it reduces the need for exchange transfusion (177,217, 462,463). Ip and associates have calculated that about 5 to 10 term and near-term infants with TSB levels between 15 and 20 mg/dL (257 to 342 μmol/L) will need to receive phototherapy in order to prevent the TSB in 1 infant from reaching 20 mg/dL (the number needed to treat) (139,140). This means that about 8 to 9 of every 10 infants with these TSB levels will not reach 20 mg/dL (342 μmol/L), even if they are not treated. As phototherapy has proven to be a generally safe procedure, however, the (unnecessary) treatment of many infants is considered appropriate in order to prevent some infants from reaching TSB levels that are considered potentially dangerous (see Phototherapy below for a discussion of its safety and complications).
The recommended TSB levels for exchange transfusion are intended to keep TSB levels below those at which kernicterus has been reported (124,139,140), although it is recognized that rare cases of kernicterus have occurred in apparently healthy infants and in sick infants at unexpectedly low TSB levels (43,124,139, 140). With rare exceptions, exchange transfusion is recommended only after intensive phototherapy has failed to keep the TSB level below the exchange transfusion level (76).
As discussed above (see section on Bilirubin Toxicity) infants (particularly those with the risk factors listed in Tables 35-29,35-30 and 35-31 and Figs. 35-22 and 35-23) (43,45,154) and preterm infants (45,150,152,464) are at a greater risk for developing kernicterus at lower bilirubin levels than are well term infants, although
some studies have not confirmed all of these associations (152,178,179). On the other hand, there is no doubt that infants of lower gestation are at a greater risk of developing high TSB levels (203,206).
Because one of the primary goals of treatment is to prevent further increases in the TSB levels, treatment is recommended at lower TSB levels at younger ages.
TABLE 35-32 GUIDELINES FOR INITIATING PHOTOTHERAPY AND EXCHANGE TRANSFUSIONS IN NICHHD NEONATAL RESEARCH NETWORK TRIAL (B. MORRIS, PERSONAL COMMUNICATION, 2002) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||
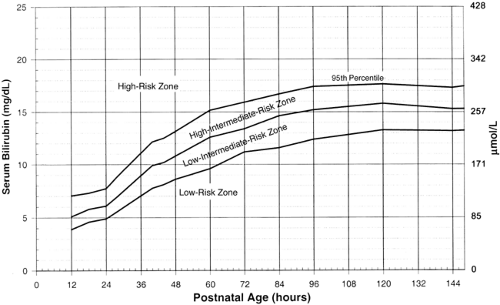 Figure 35-22 AAP guidelines for phototherapy in hospitalized infants of 35 or more weeks’ gestation (76). Note: These guidelines are based on limited evidence and the levels shown are approximations. The guidelines refer to the use of intensive phototherapy which should be used when the TSB exceeds the line indicated for each category. Infants are designated as “higher risk” because of the potential negative effects of the conditions listed on albumin binding of bilirubin (77,96), the blood-brain barrier (60), and the susceptibility of the brain cells to damage by bilirubin (55). “Intensive phototherapy” implies irradiance in the blue-green spectrum (wavelengths of approximately 430 to 490 nm) of at least 30 μW/cm2 per nm (measured at the infant’s skin directly below the center of the phototherapy unit) and delivered to as much of the infant’s surface area as possible. Note that irradiance measured below the center of the light source is much greater than that measured at the periphery. Measurements should be made with a radiometer specified by the manufacturer of the phototherapy system.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|