Fig. 5.1
POP-Q exam. Aa point A anterior, Ap point A posterior, Ba point B anterior, Bp point B posterior, C cervix or vaginal cuff, D posterior fornix (if cervix is present), gh genital hiatus, pb perineal body, tvl total vaginal length
Aa—anterior vaginal wall 3 cm proximal to the hymen
Ba—most distal position of the remaining upper anterior vaginal wall
C—most distal edge of cervix or vaginal cuff scar
D—posterior fornix (not applicable if post-hysterectomy)
Ap—posterior vaginal wall 3 cm proximal to the hymen
Bp—most distal position of the remaining upper posterior vaginal wall
Genital hiatus (gh)—middle of external urethral meatus to posterior midline hymen
Perineal body (pb)—posterior margin of gh to middle of anus
Total vaginal length (tvl)—depth of vagina with prolapse reduced
The POPQ can be categorized into stages:
Stage 0—No prolapse
Stage 1—The most distal point of the prolapse is at least 1 cm above the level of the hymen
Stage 2—The most distal point of the prolapse is between 1 cm proximal and 1 cm distal to the level of the hymen
Stage 3—The most distal point of the prolapse is between 1 cm distal to the level of the hymen and 2 cm less than the tvl
Stage 4—The most distal point of the prolapse is equal to or beyond 2 cm less than the tvl, from the level of the hymen
Some clinicians simplify the POPQ exam and do not routinely measure Aa or Ap.
Other staging systems generally utilize the relationship of the leading edge of the prolapse to the hymenal ring or introitus.
Imaging is not routinely used in the evaluation of pelvic organ prolapse. However, it has been argued that clinical examination assesses surface anatomy and is more limited in assessing structural abnormalities [5]. Underestimation or misdiagnosis of the compartment that is prolapsed can occur in 45–90 % of cases [6]. What appears to be a cystocele could in rare cases be a urethral diverticulum, Gartner duct cyst, or anterior enterocele [5]. Thus, in ambiguous cases, translabial ultrasound or dynamic MR imaging can be utilized. However, this is not routine, requires trained personnel to interpret the imaging, and may be cost-prohibitive.
Surgical Repair
The patient is placed in the dorsal lithotomy position. The external genitalia is prepped and draped in the usual sterile fashion. Some choose to shave the perineum but it is not required. One dose of intravenous antibiotics is administered for prophylaxis prior to incision. As per the 2011 American Urological Association Guidelines on Antibiotic Prophylaxis, the antibiotics of choice for vaginal surgery are a first/second generation Cephalosporin or an Aminoglycoside (Aztreonam if the patient has renal insufficiency) plus Metronidazole or Clindamycin. Alternative antibiotics are Ampicillin/Sulbactam or Flouroquinolone [7]. A foley catheter is placed to drain the bladder. A weighted speculum is placed in the vagina. A Scott retractor or translabial sutures are used to retract and expose the prolapse. The anterior compartment prolapse repair is then performed using one of the following techniques: traditional anterior colporrhaphy, mesh-augmented colporrhaphy, or paravaginal defect repair.
Traditional Anterior Colporrhaphy
1.
An Allis clamp is placed 1 cm distal to the vaginal cuff or cervix. A second Allis clamp is placed just proximal to the bladder neck.
2.
While pulling the anterior vaginal wall outward with the Allis clamps, the vaginal wall is infiltrated superficially with a dilute solution of lidocaine mixed with epinephrine (Fig. 5.2).
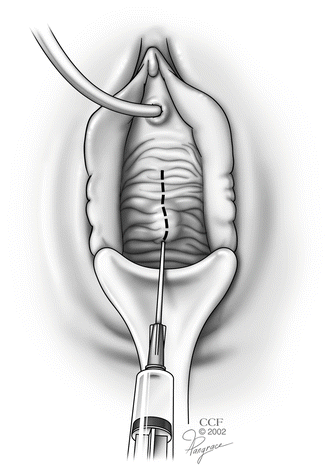

Fig. 5.2
Hydrodissection with a dilute solution of lidocaine mixed with epinephrine. (Reprinted with permission, Cleveland Clinic Center for Medical Art and Photography © 2002–2013. All Rights Reserved.)
3.
A midline incision is made between the two Allis clamps (Fig. 5.3).
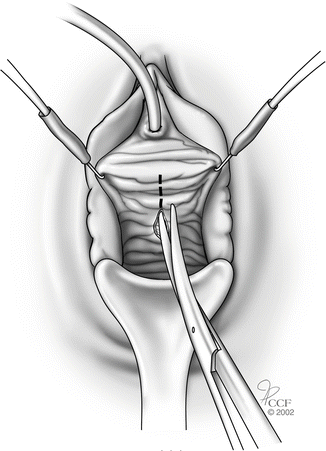

Fig. 5.3
Midline incision for anterior colporrhaphy. (Reprinted with permission, Cleveland Clinic Center for Medical Art and Photography © 2002–2013. All Rights Reserved.)
4.
Allis clamps are placed on the edges of the vaginal skin on both sides of the incision.
5.
While retracting the Allis clamp outward, the assistant should provide countertraction on the pubocervical fascia thus delineating the plane between the vaginal skin and pubocervical fascia. (Note, the more appropriate term is vaginal muscularis and not pubocervical fascia, since there is no actual fascial layer.) A combination of sharp and blunt dissection is used to dissect the vaginal skin off the underlying pubocervical fascia (Fig. 5.4).
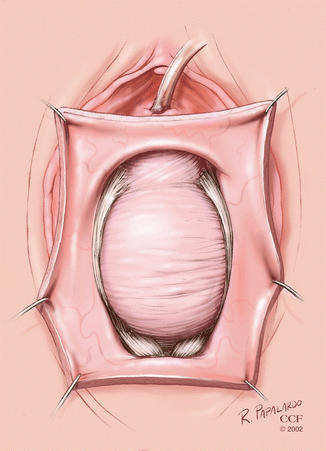

Fig. 5.4
Dissection between vaginal skin and pubocervical fascia. (Reprinted with permission, Cleveland Clinic Center for Medical Art and Photography © 2002–2013. All Rights Reserved.)
6.
The pubocervical fascia is then plicated in the midline with 2-0 absorbable interrupted sutures (Fig. 5.5).
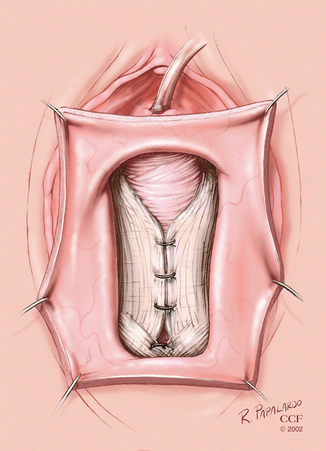

Fig. 5.5
Plication of pubocervical fascia for anterior colporrhaphy. (Reprinted with permission, Cleveland Clinic Center for Medical Art and Photography © 2002–2013. All Rights Reserved.)
7.
Many surgeons perform a cystoscopy at this point to evaluate for ureteral efflux ensuring that they have not caused ureteral obstruction, and to verify there are no sutures in the bladder.
8.
Excess vaginal skin is excised and the incision is closed with a running, locking absorbable suture—it is important not to overtrim the vaginal skin.
9.
If a concomitant mid-urethral sling is being placed, it should be done after the cystocele repair through a separate more distal incision.
Mesh-Augmented Colporrhaphy
Mesh placement for mesh-augmented anterior colporrhaphy can be performed using self-tailored biologic or synthetic mesh, a transobturator and/or transgluteal trocar-guided synthetic mesh kit, or a non-trocar synthetic mesh kit. (The objective outcome data is better for macroporous polypropylene synthetic mesh compared to biologic mesh. See section on Recent Randomized Trials on Outcomes)
1.
An Allis clamp is placed 1 cm distal to the vaginal cuff or cervix. A second Allis clamp is placed 1–2 cm proximal to the bladder neck.
2.
While pulling the anterior vaginal wall outward with the Allis clamps, the vaginal wall is infiltrated deeply and hydrodissected with a dilute solution of lidocaine mixed with epinephrine, thus developing a plane between the pubocervical fascia and the bladder adventitia.
3.
A midline incision is made between the two Allis clamps.
4.
Allis clamps are placed on the edges of the vaginal skin on both sides of the incision.
5.
A combination of sharp and blunt dissection is used to dissect the vaginal skin with the underlying pubocervical fascia off the bladder adventitia, thus developing the vesicovaginal space. The dissection to achieve this plane is very different from the traditional colporrhaphy dissection. The correct plane for mesh placement is critical to ensure that mesh extrusion does not occur.
6.
The mesh is placed loosely to allow for possible scarring and tightening of the mesh.
7.
The mesh is secured to the arcus tendineus fascia pelvis (ATFP), iliococcygeus muscle, or sacrospinous ligament depending on the technique used (Fig. 5.6).
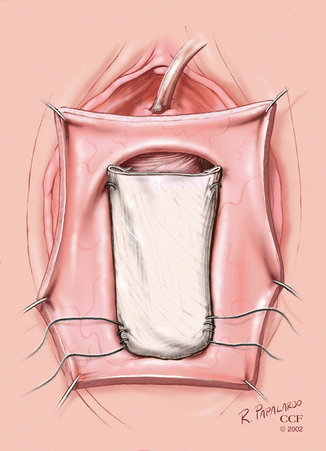

Fig. 5.6
Biologic mesh-augmented anterior colporrhaphy. (Reprinted with permission, Cleveland Clinic Center for Medical Art and Photography © 2002–2013. All Rights Reserved.)
8.
Cystoscopy is performed to verify there are no sutures or mesh in the bladder.
10.
A vaginal pack is left in place overnight
Paravaginal Defect Repair
1.
The pubocervical fascia is exposed in the same manner as detailed above for traditional anterior colporrhaphy. However, the dissection is extended further so that the anterior border of the developed space is the ischiopubic rami, the medial border is the pubic symphysis, and the lateral border is the ischial spine.
2.
The pubocervical fascia is then plicated in the midline with 2-0 absorbable interrupted sutures.
3.
The lateral defect is then repaired by placing nonabsorbable suture through the avulsed lateral edge of the pubocervical fascia, ATFP, and muscularis of the lateral vaginal wall. Transvaginally the exposure and suture placement on the arcus tendineous can be challenging but is made easier by use of a device that allows for suture placement based on palpation (Capio®, Boston Scientific Company). A series of four to six stitches are placed along the ATFP from the ischial spine toward the level of the urethrovesical junction.
4.
The same is performed on the contralateral side.
5.
The sutures are tied sequentially from one side to the other starting from the urethrovesical junction heading toward the ischial spine. This technique can also be performed transabdominally. Transabdominal paravaginal defect repair is currently often performed with a minimally invasive laparoscopic or robotic approach.
6.
Cystoscopy is performed to evaluate for ureteral efflux and to verify there are no sutures in the bladder.
7.
Excess vaginal skin is excised and the incision is then closed with a running, locking absorbable suture.
Recent Randomized Trials on Outcomes [9]
Several prospective trials have compared outcomes between these approaches. The first randomized trial comparing anterior colporrhaphy techniques was published by Weber et al. in 2001 [10]. Chmielewski et al. reanalyzed the data using a more clinically relevant definition of success, which included no prolapse beyond the hymen, absence of prolapse symptoms, and absence of retreatment. One hundred fourteen women were randomized to standard anterior colporrhaphy, ultralateral colporrhaphy, or anterior colporrhaphy with (absorbable) mesh, and were followed at 6 months, 1 year, and 2 years after repair. Eighty-eight percent of women with sufficient follow-up data at 1 year met the definition of surgical success. There was no difference between repair groups. The authors concluded that standard anterior colporrhaphy is appropriate for primary cystocele repair at 2 years follow-up [11]. Some have criticized this study as a reanalysis years after the original with changing definitions and relatively short follow-up given the length of time from the original study [12].
Several more recent randomized, controlled trials have compared anterior colporrhaphy with mesh-augmented colporrhaphy. Anatomical success rates for anterior colporrhaphy were 41–72 % versus 81–91 % for self-tailored synthetic mesh-augmented colporrhaphy and 87–91 % for synthetic mesh-kit-augmented colporrhaphy at 1 year follow-up. Vaginal mesh extrusion rates were 4–7 %. Rates of de novo dyspareunia were not significantly different between groups [13–16].
The randomized, controlled study with the longest follow-up, 3 years, reported 59 % anatomical success in the traditional colporrhaphy group versus 87 % in the synthetic mesh-augmented colporrhaphy group (p < 0.0001). Symptomatic outcomes and rates of de novo dyspareunia were similar in both groups. The mesh extrusion rate was 19 % [17].
The largest randomized controlled trial compared 389 women who underwent trocar-guided, transvaginal polypropylene-mesh repair (Gynecare Prolift™ Anterior Pelvic Floor Repair System kit, Ethicon) to women who underwent traditional colporrhaphy. The primary outcome was a composite of anatomic and symptomatic success. At 1-year follow-up, 60.8 % of women treated with transvaginal mesh had no prolapse or vaginal bulge symptoms compared to 34.5 % of women who underwent traditional colporrhaphy (p < 0.001). The rate of reoperation for mesh exposure was 3.2 %. The mesh repair group also experienced more surgical complications such as longer operating time, increased blood loss, bladder injury, and mesh-related complications [18].
A criticism of several studies has been that anterior colporrhaphy has had worse anatomic outcomes compared to mesh-augmented colporrhaphy due to not addressing concomitant apical prolapse. Nguyen et al. performed a randomized trial comparing anterior colporrhaphy with synthetic mesh-augmented colporrhaphy. The majority of these women underwent uterosacral vault suspension. At 1-year follow-up, anatomic success (no stage 2 or greater anterior prolapse) was 55 % and 87 % in the traditional and synthetic mesh-augmented groups, thus demonstrating the superiority of mesh-augmented repairs even after addressing apical prolapse in both groups [15].
Given the risk of extrusion with synthetic mesh, three randomized trials have compared traditional colporrhaphy with biologic graft-augmented colporrhaphy. Meschia et al. utilized Pelvicol in their study of women with primary stage 2 or greater cystocele. At 2 years follow-up, the failure rate in the traditional group was 23 % versus 11 % in the Pelvicol group (RR 2.08, 95 % CI 1.08–4.01). One woman underwent graft removal due to “rejection.” Subjective outcomes at 2 years were not reported [19]. Dahlgren et al. performed a similar study utilizing Pelvicol® (Bard Medical) for women with recurrent cystocele. Recurrence rates at 3 years follow-up were 57 % without versus 62 % with Pelvicol (not significant). Symptomatic improvement and sexual function were similar in both groups [20]. The third randomized trial utilized cadaveric fascial lata (Tutoplast®, Davol) in women with primary or recurrent cystocele. At 13 months follow-up, there was no difference in objective and subjective outcomes [19]. Thus, it seems there is no functional or symptomatic advantage with the use of a biologic graft for colporrhaphy.
A randomized controlled trial comparing traditional colporrhaphy, xenograft-augmented, and synthetic mesh-augmented colporrhaphy has been conducted with 2 year follow-up. Anatomic failure rate was 58 %, 46 %, and 18 % in each respective group (p < 0.05). Symptomatic failure rates were not statistically different between groups. The mesh extrusion rate was 14 %. The authors concluded that synthetic mesh-augmented repair had the best anatomic outcome but symptomatic outcomes were similar between all groups [21].
Finally, a recent Cochrane review has noted that there are better outcomes for anterior repair with mesh augmentation than anterior colporrhaphy alone and that synthetic mesh does better than biologic mesh [19].
See Table 5.1.
Table 5.1
Success rates for pelvic organ prolapse repair
Objective success (%) | Subjective success (%)a | |
|---|---|---|
Anterior repair | ||
Traditional | 41–89 | 81–100 |
Paravaginal | 83 | 93 |
Synthetic mesh augmented | 81–96 | 81–91 |
Biologic mesh augmented | 54–82 | |
Posterior repair | ||
Traditional | 75–91 | 80–93 |
Site-specific | 78 | 88 |
Synthetic mesh augmented | 78–96 | 79–96 |
Biologic mesh augmented | 54–88 | 97 |
Apical repair | ||
USVS | 86–97 | 94 |
SSLF | 69 | 91 |
Transvaginal mesh augmented | 43 | 79 |
Open ASC | 76–94 | 94 |
Laparoscopic ASC | 77–91 | 87 |
Robotic ASC | 88–94 | |
Uterine sparing ASC | 68 | |
Hysterectomy and A–P repair | 87 | |
Colpocleisis | 85–100 | |
Summary
The randomized controlled trials comparing colporrhaphy with and without mesh are heterogeneous. However, the consensus seems to be that mesh repair provides superior anatomic outcome but equivalent symptomatic outcome. While subjective outcomes appear similar in the short run, one cannot know at this point if that will remain so in the long run. Specifically, will those patients with asymptomatic anatomic recurrences now become those that have symptomatic recurrences in the future? Rates of de novo dyspareunia are not significantly increased with mesh repair, but mesh exposure rates and the increased surgical complication rates are not negligible. Given the risk of mesh extrusion, the FDA safety update on transvaginal mesh for pelvic organ prolapse [22], and after a recent $5.5 million settlement against the manufacturers of Avaulta mesh, surgeons must use caution when utilizing transvaginal mesh [23]. Does that mean there is no role for the use of synthetic mesh in transvaginal cystocele repair? The reality is that a percentage of women will fail primary traditional colporrhaphy and women with Stage 3 or higher prolapse are more likely to recur [24]. We thus recommend a pragmatic approach to the use of transvaginal mesh for prolapse repair [25], by carefully selecting women at high risk for recurrence and having an informed discussion of the postoperative risks and complications of synthetic mesh-augmented colporrhaphy. In addition, some women with significant apical prolapse with concomitant anterior vaginal wall prolapse may do well with an abdominal sacrocolpopexy. This is discussed further in the section on apical prolapse.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


