Fig. 1
This 5-week-old infant has his head tilted to the left and chin slightly rotated towards the right in the typical “cock robin” position that is characteristic of congenital muscular torticollis
Congenital muscular torticollis is considered to be an in utero packaging problem, and therefore a pertinent history can reveal specific risk factors including breech positioning and primiparous (first born) birth. In a prospective series of 1,086 cases, 13 % had a breech presentation (Cheng et al. 2000), and the male to female ratio was three to two. While it is typically felt that the right side is more often affected, in this large series, there was no predilection towards either side (Cheng et al. 2000). Packaging disorders including sonographic hip dysplasia are found in up to 20 % of patients (Hummer and MacEwen 1972; Morrison and MacEwen 1982; Cheng et al. 2000) and foot deformities in 6.5 % (Cheng et al. 2000). While a thorough physical examination of the hip is absolutely mandatory for signs of hip dysplasia, an argument can also be made for sonographic hip screening in all patients with CMT.
Congenital muscular torticollis must be carefully differentiated from nonmuscular causes (Table 1). Up to 18 % of torticollis can be attributed to nonmuscular etiologies (Ballock and Song 1996). These include congenital cervical vertebral anomalies such as those seen in Klippel-Feil and postinfectious atlantoaxial rotatory displacement (Grisel syndrome ). Other causes include posttraumatic or postinflammatory upper cervical rotatory instability as seen in patients with juvenile idiopathic arthritis. Neoplastic conditions; ocular causes including paralytic strabismus (Kushner 1979); neurologic causes such as posterior fossa tumors, syringomyelia, and Arnold-Chiari malformations; and Sandifer syndrome (spasmodic torticollis associated with GERD) must also be considered. Nonmuscular causes of torticollis typically present much later than congenital muscular torticollis.
Table 1
Differential diagnosis of torticollis
Congenital vertebral anomalies |
Postinfectious (Grisel syndrome) |
Inflammatory/juvenile idiopathic arthritis |
Traumatic |
Neoplastic |
Posterior fossa tumors |
Cervical spine tumors |
Ocular (e.g., paralytic strabismus) |
Neurologic |
Syringomyelia |
Arnold-Chiari malformation |
Sandifer syndrome |
Pathoanatomy and Applied Anatomy
The main culprit in congenital muscular torticollis appears to be a fibrotic and shortened sternocleidomastoid muscle. The SCM muscle consists of two heads: sternal and clavicular. The two heads quickly coalesce into one muscle belly and insert on the lateral aspect of the mastoid process of the temporal bone (Fig. 2). The SCM functions to rotate the head towards the opposite side and to tilt the head towards the affected side. Patients with CMT have a shortened or fibrotic SCM: the typical head position is tilted towards and rotated away from the affected side. The motor innervation of the SCM is supplied by the ipsilateral spinal accessory nerve that travels in close proximity to the muscle. The spinal accessory nerve approaches the SCM at its anterior margin approximately 3 cm distal to the mastoid process in adult patients. It continues in a cranial to caudal direction along the ventral surface of the SCM in approximately 50 % of patients and partially pierces the muscle in approximately 50 % of cases continuing dorsolaterally (Lee et al. 2009). At the posterior border of the SCM, the nerve crosses between one-third and two-thirds of the junction of the muscle when its length is measured from the mastoid process to the sternal attachment (Chen et al. 2009). Care must be taken to avoid injury to the spinal accessory nerve when a proximal SCM release is being performed.
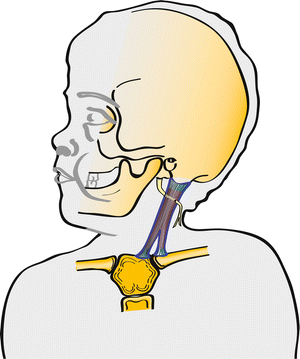

Fig. 2
The sternocleidomastoid muscle consists of a sternal and clavicular head that coalesce into one muscle belly before inserting on the mastoid process of the temporal bone. Notice that the spinal accessory nerve travels deep to the muscle and lies at one-third to two-thirds of the junction when measuring the muscle from the mastoid process to the sternal attachment. In 50 % of cases the nerve pierces the muscle
Assessment of Torticollis
Signs and Symptoms
Head tilt is often the primary indicator of CMT. The head is noted to be tilted towards and rotated away from the affected side. In some cases, translation of the entire head towards the opposite side can be seen (Fig. 3). Confirmation of the SCM contracture is performed by examining the passive range of motion of the head and neck. Head tilt and head and neck rotation to the right and left are both measured. Examination of rotation should be performed in a supine position on a flat surface with the head of the child over the edge such that the head and neck are completely free to rotate without hindrance. A two-person technique is ideal as the shoulders can be stabilized by one person, while the head and neck rotation is performed and measured by the other. The measurement can be made with either a goniometer or a specialized protractor (Fig. 4). Both passive and active rotation of the head and neck towards the affected side will be decreased (Fig. 5). Similarly, head tilt towards the unaffected side will also be decreased. Placing the SCM under mild tension by holding the head in slight extension can facilitate identification of the contracted side. In certain instances, passive range of motion can be symmetric signifying that the SCM is not shortened. When torticollis exists without a restricted passive range of motion, the condition can be referred to as being postural.
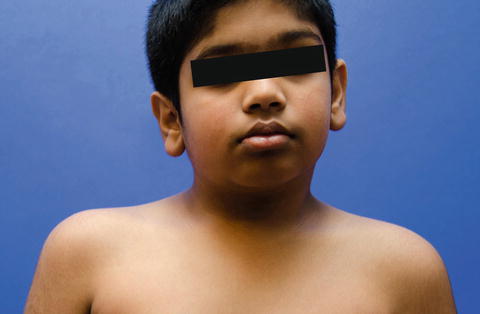
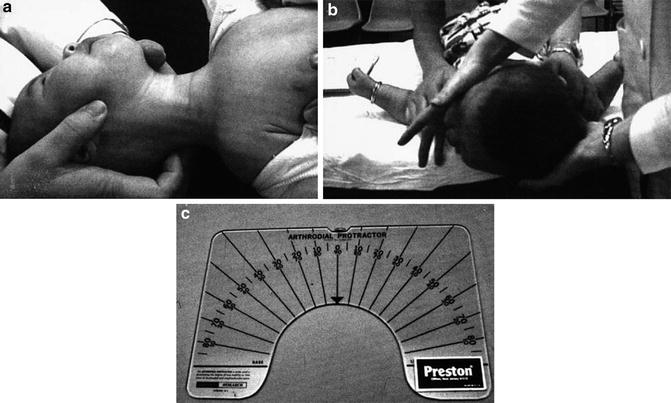
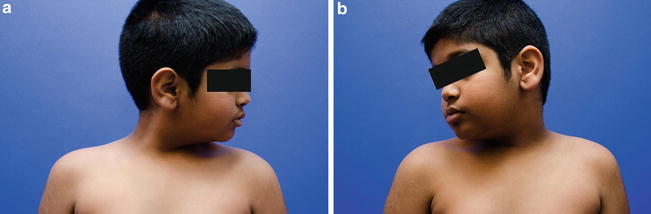

Fig. 3
The head is tilted and translated to the left and rotated to the right in this 10-year-old male with persistent CMT

Fig. 4
(a, b) Proper positioning of the neck for examination and measurement of passive rotation. (c) Arthrodial protractor (Reprinted with permission from “The clinical presentation and outcome of treatment of congenital muscular torticollis in infants – a study of 1,086 cases” by Cheng et al. 2000. J Pediatr Surg, 35(7), 1091–6, Copyright (2013) by Elsevier)

Fig. 5
Notice that rotation towards the affected (left) side is normal (a) but that rotation towards the right side (b) is decreased
Of patients with a congenital muscular torticollis, some will present with a firm mass or pseudotumor within the SCM. The incidence of developing an SCM pseudotumor of infancy is approximately 0.4 % of live births (Coventry and Harris 1959; Thomsen and Koltai 1989). When present, the pseudotumor usually appears between 2 and 4 weeks of life and typically measures between 1 and 3 cm in its largest dimension. It is generally located in the distal third of the SCM and it gradually regresses by around 2–6 months (Macdonald 1969; Morrison and MacEwen 1982) to be replaced by a fibrous band. The torticollis can become more pronounced as the initial mass regresses to form the fibrous band. The pseudotumor is also known as fibromatous colli seeing as histologic sections of the pseudotumor reveal fibromatous changes (Chandler and Altenberg 1944; Lidge et al. 1957; Morrison and MacEwen 1982) and fine needle aspirate cytology shows fibroblasts, multinucleated giant cells and atrophic muscle fibers (Kumar and Pradhan 2011). It is postulated that the mass develops secondary to an ischemic event or compartment syndrome of the SCM due to intrauterine crowding or perinatal trauma (Davids et al. 1993) that eventually leads to an SCM contracture.
Plagiocephaly is present in the vast majority of patients with up to 94 % affected at first presentation (Canale et al. 1982; Cheng et al. 2000). Early on, the asymmetry is typically mild; however, severe asymmetry is noted in up to 10 % of cases at presentation (Cheng et al. 2000). Plagiocephaly is an asymmetric flattening of the skull secondary to external forces. Supine positioning in patients with SCM contractures often results in plagiocephaly on the contralateral side, while prone positioning can result in ipsilateral facial flattening (Fig. 6). Plagiocephaly typically worsens in the first few months of life during which time head control is limited and head positioning is strongly influenced by any muscular asymmetry. As the child gains active control of the neck, plagiocephaly decreases; however, it can persist and may require treatment in the form of counter positioning therapy and in more severe cases cranial molding orthoses.

Fig. 6
Plagiocephaly can result from CMT due to supine or prone positioning in a favored direction. Supine positioning results in contralateral posterior skull flattening, while prone positioning results in ipsilateral facial flattening when associated with CMT
Facial asymmetry is typically not present early on; however, it can develop in untreated muscular torticollis. Facial bone morphologic changes appear after 5 years of age corroborating evidence that early treatment can prevent and reverse facial asymmetry that develops in long-standing untreated congenital muscular torticollis (Canale et al. 1982; Yu et al. 2004). Characteristics of facial asymmetry include downward sloping of the ipsilateral eye, ear, and nose and more specifically can be described as “recessed eyebrow and zygoma, deviation of the chin point and nasal tip, inferior orbital dystopia on the affected side, commissural canting toward the affected side, inferiorly and posteriorly positioned ipilateral ear, and distorted craniofacial skeletal structures” (Yu et al. 2004; Fig. 7).
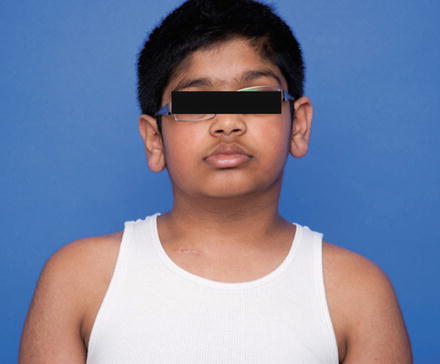

Fig. 7
This photo demonstrates facial asymmetry in an older child with persistent CMT that was not surgically addressed until age 10. Notice specifically the inferiorly positioned ear and inferior orbital dystopia on the affected side
In addition to assessing the torticollis itself, a general examination must be performed to rule out associated packaging disorders including hip dysplasia and foot deformities. In the older child, adolescent, or adult patients with recurrent or previously untreated CMT, shoulder asymmetry with or without a mild upper thoracic scoliosis may be noted (Fig. 8). They are likely related to both the contracture of the SCM and the compensation for the head tilt and can become structural. The scoliotic deformity does not typically require treatment.
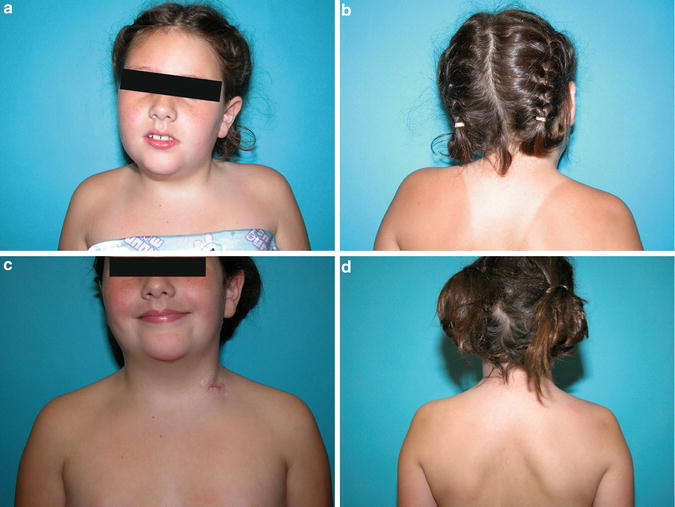

Fig. 8
In addition to obvious head tilt and rotation (a), elevation of the left shoulder (b) is noted in this 9-year-old female with persistent CMT. A bipolar release was effective in adequately releasing the tight sternocleidomastoid muscle to eliminate head tilt and rotation (c) as well as the shoulder asymmetry (d)
Imaging and Other Diagnostic Studies
Imaging for CM T is generally limited in the presence of the typical SCM contracture especially since radiographs can be both difficult to obtain and difficult to interpret during infancy. In the absence of the expected range of motion restriction or in cases unresponsive to conservative treatment, cervical spine X-rays are warranted to rule out osseous abnormalities such as those seen in Klippel-Feil syndrome . Ultrasound (US) and MRI have both been used to assess the SCM pseudotumor; however, routine use for the typical presentation is not warranted. Atypical presentation or unresponsive cases with normal X-rays warrant further diagnostic studies including US and/or MRI. In unresponsive or atypical cases, US should be the next line of investigation when a pseudotumor is present, whereas, in the absence of a pseudotumor, an MRI is the investigation of choice.
Ultrasound
Sonographic appearance can vary from diffuse enlargement of the muscle with mixed echogenicity to a discrete hyperechoic mass within the SCM (Bedi et al. 1998; Fig. 9). As long as the abnormality is intramuscular and the adjacent soft tissues are normal, the diagnosis is readily made despite its variable appearance. Routine use of ultrasound is not necessary; however, in cases of suspected SCM pseudotumor where the mass is large (>3 cm), expansile, presents late (>3 months), or the range of motion and head positioning are inconsistent with congenital muscular torticollis, US can be useful to rule out other causes of a neck mass.
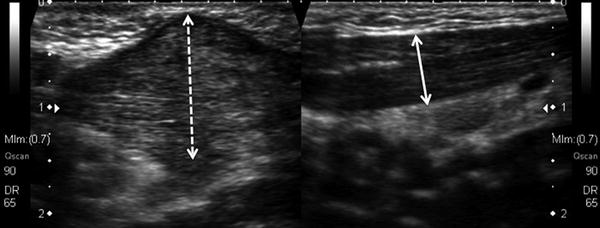

Fig. 9
Sagittal ultrasound images of the neck reveal a heterogenous and relatively isoechoic mass (dashed white line) measuring 2.3 × 1.3 × 2 cm within the SCM muscle (a) in a 30-day-old male that presented with torticollis and an SCM pseudotumor as compared to the normal side (b) demonstrating uniform width of the SCM muscle (solid white line). The sonographic appearance of the pseudotumor can vary markedly from patient to patient
CT
CT scans are not indicated when CMT is suspected; however, they are useful for detecting congenital bony malformations of the occipitocervical junction that may result in a nonmuscular torticollis that may not be obvious on plain radiographs (Fig. 10).
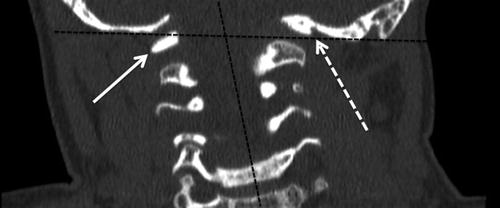

Fig. 10
A coronal CT image of the cervical spine in a 3-year-old patient that presented with a head tilt revealed formation and segmentation anomalies of the atlas. Angulation between the head and neck is noted (dashed black lines) in addition to an occipito-C1 fusion anomaly and C1 hypoplasia on the left side (dashed white arrow) with relatively normal formation of the right side of C1 (solid white arrow) resulting in head tilt
MRI
MRI is of limited diagnostic utility and is not recommended for suspected CMT (Parikh et al. 2004). However, MRI is useful for the detection of nonmuscular causes of torticollis including neurogenic and osseous abnormalities. MRI is recommended only when the clinical symptoms of a suspected CMT are not responsive to standard treatment and a nonmuscular cause is being considered.
Classification
Congenital muscular torticollis can be classified into three groups: CMT with pseudotumor, CMT without pseudotumor, and positional torticollis. Positional torticollis presents with a similar appearance as the first two in terms of head position; however, patients have a normal and symmetric range of motion.
Outcome Tools
Important features determining the outcome of treatment for congenital muscular torticollis include the presence of head tilt, restriction in range of motion, severity of facial asymmetry, and severity of plagiocephaly. Two outcome tools have been developed although they have not been validated or tested for reliability. Both tools use a number of subjective parameters due to the lack of objective parameters to reliably measure craniofacial asymmetry. Recently, objectively measurable radiographic craniofacial curvature and asymmetry parameters have been used to evaluate surgical outcomes although they have not yet been incorporated into any outcome tools.
Canale et al. developed a categorical grading (Table 2) for outcomes based on combining cosmetic and functional results that were individually graded as satisfactory or unsatisfactory (Canale et al. 1982). Cosmetic results are graded as satisfactory when the patient has no facial asymmetry or facial asymmetry noted only by the examiner, no head tilt, and no palpable tightness of the SCM. A cosmetic result is deemed unsatisfactory when the patient has facial asymmetry that is apparent to the parents or the patient or both, and there is a residual deformity of the SCM, with or without head tilt. Functional results are graded satisfactory when the patient has full rotation of the head or loss of rotation of less than 30°, while an unsatisfactory functional result occurs when the patient has a loss of rotation of 30° or more, with or without discomfort. The cosmetic and functional results are combined to form three groups categorized as good, fair, and poor. Group 1 consists of patients with a satisfactory functional and cosmetic result and is considered a good outcome;
Table 2
Canale grading scheme for outcome of torticollis treatment. Function is deemed satisfactory when loss of rotation is <30°. Cosmesis is satisfactory when there is no facial asymmetry noticed by the patient or family, no head tilt, and no palpable SCM tightness
Function | Cosmesis | |
|---|---|---|
Group 1 (good) | Satisfactory | Satisfactory |
Group 2 (fair) | Satisfactory | Unsatisfactory |
Group 2 (fair) | Unsatisfactory | Satisfactory |
Group 3 (poor) | Unsatisfactory | Unsatisfactory |
Group 2 includes patients with an unsatisfactory functional or cosmetic result and is considered to have a fair outcome; and Group 3 has unsatisfactory functional and cosmetic results and is considered to have a poor result.
More recently Cheng et al. developed a torticollis assessment score (Table 3; Cheng et al. 2001) based on a previous scoring system (Lee et al. 1986). They assign a score of 0 to 3 for each of six features including rotational deficit, lateral bending, craniofacial asymmetry, residual SCM band, head tilt, and a subjective cosmetic and functional assessment by the parents. The outcome is considered to be excellent when the score is 16–18, good when 12–15, fair when 6–11, and poor when <6 (Cheng et al. 2001).
Score | ||||
|---|---|---|---|---|
Category | 3 points | 2 points | 1 point | 0 points |
Rotation deficit (°) | <5 | 6–10 | 11–15 | >15 |
Lateral bending deficit (°) | <5 | 6–10 | 11–15 | >15 |
Residual band (none, lateral, cleidal, or sternal) | None | Mild | Moderate | Severe |
Head tilt (none, lateral, cleidal, or sternal) | None | Lateral | Lateral, cleidal | Cleidal, sternal |
Subjective assessment by parents (cosmetic and functional) | None | Mild | Moderate | Severe |
Excellent | Good | Fair | Poor | |
Efforts to quantify postsurgical craniofacial deformity improvement in patients with CMT based on radiographic craniofacial curvature and asymmetry parameters (Lee et al. 2012) have been developed. While these parameters appear effective at objectively quantifying craniofacial asymmetry, it is unlikely that they will be utilized other than for research purposes.
Torticollis Treatment Options
Nonoperative Management of Torticollis
Nonoperative management is indicated in all patients that present with congenital muscular torticollis for the first time and in those that have not had adequate treatment.
Congenital muscular torticollis | |
|---|---|
Nonoperative management | |
Indications | Contraindications |
First time presentation of CMT | Nonmuscular causes of torticollis |
Previous inadequate trial of nonoperative management | |
Nonoperative treatment has a success rate of approximately 90 % when initiated before the age of 1 (Cheng et al. 2000, 2001). The standard treatment consists of physiotherapy that includes a home therapy program centered on active movement stimulation, manual stretching, and, in cases of plagiocephaly, counter-positioning. Additional nonoperative treatment options include the use of botulinum toxin A in select cases.
Physiotherapy for CMT is centered on stretching the affected SCM through both passive and active means. Manual stretches, active stimulation, and counter-positioning are taught to the parents. Formal visits with the physiotherapist every 2 weeks ensure that the exercises are being performed correctly and enable monitoring of improvement. Active stimulation involves having the child follow toys towards the left and right sides and having the child actively stretch the contracted SCM by rotating towards the affected side. Manual stretching is recommended at each diaper change with a minimum of two sessions daily. The stretches are to be held for approximately 10–15 s and each stretch is to be repeated three to five times. While some physiotherapists advocate a manual stretch that combines head tilt away from the contracted SCM and rotation towards the affected side, others recommend performing these exercises separately (Fig. 11). Counter-positioning can be performed in supine, prone, and even sitting positions to further promote stretching of the SCM and involves positioning toys and other distractions on the affected side (Fig. 12). In cases of plagiocephaly, increasing prone awake time and alternating sides while supine is recommended to decrease the time spent on the side of the plagiocephaly.
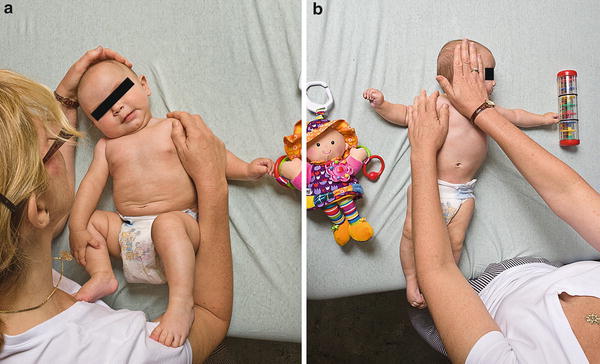
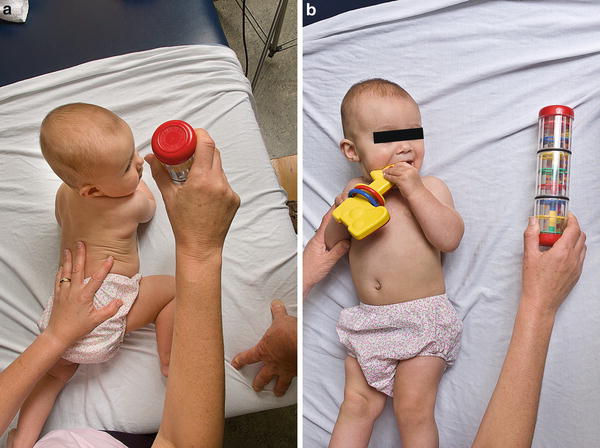

Fig. 11
Tilt stretches (a) and lateral rotation stretches (b) are performed by parents three to five times a day. The position is held for 10–15 s and repeated three to five times per session. These movements can also be combined into one stretch

Fig. 12
Counter-positioning consists of placing toys or other distractions on the affected side to promote active stretching of the tight SCM in both the prone (a) and supine (b) positions
The overall short- and long-term outcomes of nonoperative management in the form of physiotherapy are good to excellent in about 90–95 % of cases with approximately 5 % requiring surgery when treatment is initiated during infancy (Cheng et al. 2000). Limited data is available on the outcome of nonoperative management in children older than 1. Age at presentation is an important risk factor in the effectiveness of physical therapy (Cheng et al. 2000), and while improvements can be expected in older children, the results are not as good with the probability of requiring surgical interventions increasing. Other important risk factors include the type of CMT with fair to poor results in 12.2 % of those with a pseudotumor, 6.1 % in those with a contracture alone, and 1 % in those with postural torticollis. Severity of rotational deficit at presentation also predicts failure (Cheng et al. 2000).
Botulinum toxin A is a neurotoxin that inhibits acetylcholine release and results in temporary muscle paralysis. Its use in treating muscular dystonia and spasticity has become widespread, and recently it has been advocated for certain cases of muscular torticollis. Three small retrospective studies have looked at its utility in treating recalcitrant or late presentation congenital muscular torticollis and have reported successful treatment (Joyce and de Chalain 2005; Oleszek et al. 2005; Collins and Jankovic 2006). The protocol used by Joyce et al. entailed delivery of 25–50 IU of Botox (Allergan Inc., Philadelphia, PA) depending on the weight of the patient under a general anesthesia in three to four locations evenly distributed along the SCM. This was followed by a manual stretching program of three to five stretches at each diaper change for the next 3 months (Joyce and de Chalain 2005). While long-term follow-up studies are still required on the maintenance of the correction, there appears to be some benefit to using botulinum toxin A in the treatment of young patients with recalcitrant CMT. It can be considered as the next step in the treatment algorithm prior to embarking on surgical methods. Of note, the US Food and Drug Administration performed a systematic review of adverse events related to botulinum toxins that revealed nine deaths in pediatric patients (Apkon and Cassidy 2010). In the USA, botulinum toxin A is not approved for use in children younger than 12 years of age. However, it is used ubiquitously to treat spasticity in children in an off-label fashion.
Operative Treatment of Torticollis
Surgical management of congenital muscular torticollis is indicated for all patients with persistent head tilt and passive rotation/tilt deficit >15° despite an adequate trial of physiotherapy and manual stretching exercises. In children presenting before age 1, 6 months of physiotherapy and stretching exercises should be attempted prior to surgical release. In children 5 years of age or older, if progress is not seen after 6 weeks of therapy, one may consider operative management.
Congenital muscular torticollis | |
|---|---|
Operative management | |
Indications | Contraindications |
Persistent head tilt + one of the following: | Inadequate trial of nonoperative treatment |
Passive rotation deficit > 15° | |
Passive tilt deficit >15° | |
Surgical Procedures
Surgical release of the SCM can effectively treat a recalcitrant or late presentation CMT that has failed conservative management. Standard procedures include a unipolar distal release and a bipolar release with or without distal Z-lengthening.
Preoperative planning: Prior to proceeding with surgical treatment, it is imperative to confirm the diagnosis of CMT. If there is any doubt in the diagnosis, a thorough ocular and neurologic exam should be repeated and a further workup consisting of radiographs to rule out osseous abnormalities and/or MRI to rule out neurogenic causes should be performed.
Unipolar Distal Release
Positioning: The patients should be positioned supine with the head extended and turned to the contralateral side to increase visualization of the SCM. The ear can either be included in the draped field or the ear lobe can be taped such that prepping and draping can include the mastoid process to ensure adequate visualization of the entire SCM. Ensure that the anesthetic team is aware that you may be manipulating the head during the case.
Surgical approach: Supraclavicular between the two heads of the SCM.
Technique: A 4–5 cm transverse incision 1 fingerbreadth proximal to the superior border of the clavicle centered between the sternal and clavicular heads of the SCM is made. Incise the platysma (if present) and expose the two heads of the SCM. The two heads are then dissected free and a 1–2 cm section is resected to completely section the tendons (Fig. 13). The head is manipulated to put the SCM on full stretch, and any palpable tight bands of the platysma or adjacent fascia are incised under direct vision. The subcutaneous tissues and skin are closed.
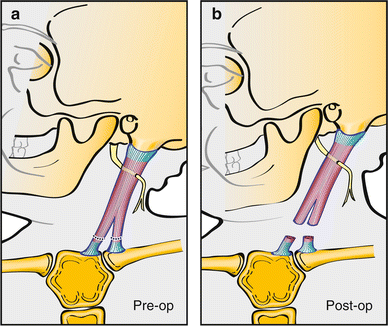

Fig. 13
The clavicular and sternal insertions are incised and a 1–2 cm portion of the tendons can be excised to ensure complete sectioning when performing a distal unipolar release
Congenital muscular torticollis |
|---|
Preoperative planning for all procedures |
OR table: any table for supine position |
Positioning/positioning aids: supine with the head extended and rotated away from the operative side |
Equipment: basic retractors and instruments |
Unipolar distal release for congenital muscular torticollis |
|---|
Surgical steps |
4–5 cm incision 1 finger breadth above the clavicle centered between the heads of the SCM |
Incise platysma |
Isolate clavicular and sternal heads |
Excise 1–2 cm portion of the SCM heads |
Manipulate the head to place SCM under tension |
Release any residual tight bands in platysma or adjacent fascia |
Close subcutaneous and skin layers |
Bipolar Release with Distal Z-Lengthening or Distal Transfer
Preoperative planning: Same as for unipolar distal release.
Positioning: Same as for unipolar distal release. The posterior hairline will likely need to be shaved to approximately mid ear level on the ipsilateral side to enable access to the mastoid insertion of the SCM.
Surgical approaches: Same as for unipolar distal release. In addition, a posterior auricular incision will be utilized to approach the proximal SCM. The spinal accessory nerve travels from anterior to posterior on the ventral surface of the SCM and sometimes pierces the muscle to exit at its dorsal and posterior margin. The nerve in adults is 3 cm from the mastoid process. Staying near the mastoid with the dissection prevents injury to the nerve.
Technique: A 3 cm incision is made posterior to the ear near the tip of the mastoid process. Bluntly dissect around the SCM just distal to the mastoid process. Pass a Penrose drain around the SCM to help deliver it out of the wound. Divide the SCM layer by layer using either scissors or electrocautery at a low setting. Typically, the spinal accessory nerve is not seen; however, if it is encountered, dissect around it and keep it protected when transecting the SCM. Next, make a 4–5 cm transverse incision 1 fingerbreadth proximal to the superior border of the clavicle centered between the sternal and clavicular heads of the SCM. Incise the platysma and expose the two heads of the SCM. Once exposed, the clavicular head is incised. A 2–3 cm longitudinal incision is then made in the sternal head, and a Z-cut of the sternal head is performed (Fig. 14). The head is manipulated to put the SCM on full stretch, and any palpable tight bands of the platysma or adjacent fascia should be incised under direct vision. An end-to-end or side-to-side repair of the sternal head is performed in a slightly overcorrected position. Finally, the subcutaneous tissues and skin are closed.
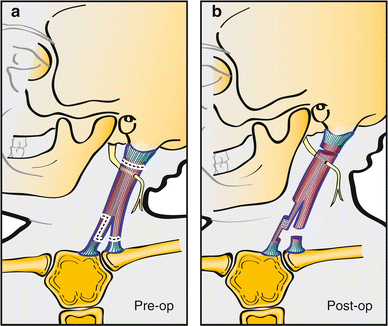

Fig. 14
Z-lengthening of the sternal head can be performed in order to maintain the normal “V” contour of the neck. A proximal unipolar release is added to further lengthen the SCM when performing a bipolar distal Z-lengthening
A modification of the Z-lengthening is a transfer of the distal clavicular head to the mid-sternal head to create a longer sternal column (Fig. 15). The idea behind both the Z-lengthening and the distal transfer is to improve appearance by maintaining the “V” contour of the neck.
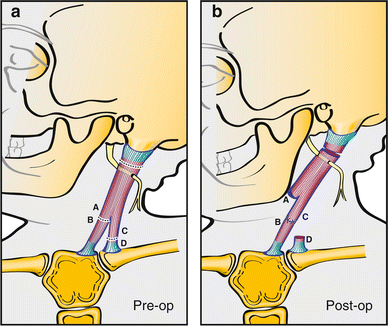

Fig. 15
An alternative to the bipolar distal Z-lengthening is a bipolar distal transfer that consists of transferring the distal end of the clavicular head to the sternal head at a slightly more proximal location. A more robust repair is feasible than when performing a Z-lengthening and the “V” contour is maintained
A bipolar lengthening procedure could be performed with a distal resection instead of a Z-lengthening or a tendon transfer (Fig. 16). And finally, an open proximal unipolar release alone could be performed although this is typically not performed due to the risk of spinal accessory nerve injury.
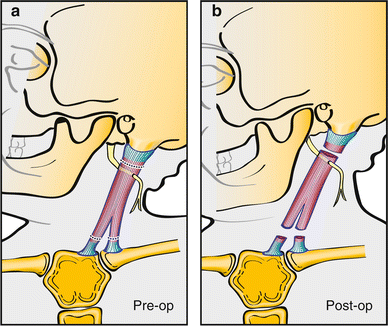

Fig. 16
Bipolar release with distal resection can be performed although it may result in the loss of the “V” contour of the neck
Bipolar release with distal Z-lengthening for congenital muscular torticollis |
|---|
Surgical steps |
3 cm incision over the proximal SCM at distal aspect of mastoid process |
Isolate the SCM using blunt dissection |
Transect the SCM close to its origin on the mastoid process (watch for spinal accessory nerve) |
4–5 cm incision 1 finger breadth above the clavicle centered between the heads of the SCM |
Incise platysma |
Isolate clavicular and sternal heads |
Incise the clavicular head |
Perform a 2–3 cm Z-lengthening cut of the sternal head |
Manipulate the head to place SCM under tension |
Release any residual tight bands in platysma or adjacent fascia |
Reapproximate the Z-cut sternal head by end-to-end or side-to-side repair with the head positioned with the SCM at full stretch |
Close subcutaneous and skin layers |
Postoperative Protocols
Postoperative protocols vary from using a soft collar with initiation of physiotherapy once the wounds have healed to application of a Minerva cast for 6–12 weeks followed by physiotherapy. In patients that are unable to participate in active and passive movement physiotherapy, casting or bracing is recommended to maintain the correction achieved; however, in those that can participate in physiotherapy, only a soft cervical collar is required. Physiotherapy is generally recommended for 4–6 weeks.
Treatment Specific Outcomes
Distal Lengthening Versus Resection
Lengthening of the SCM with resection rather than Z-lengthening is associated with the loss of the normal sternomastoid column or “V” contour of the neck. Ferkel et al. described this in 6 out of 10 patients that were treated with a tenotomy rather than a lengthening (Ferkel et al. 1983). To prevent this loss of contour, a Z-lengthening is recommended over a simple tenotomy at the level of the distal release. Similarly, a transfer of the clavicular head to the sternal head can be used to maintain the “V” contour of the neck.
Bipolar Release
Ferkel et al. reported a 92 % good or excellent outcome using the bipolar release with distal Z-lengthening compared to 15 % good results using other surgical methods including unipolar distal release without Z-lengthening (Ferkel et al. 1983). In another study looking at children older than 6, good to excellent results were noted with a bipolar release in 56 % of patients (Lee et al. 1986). Just as the success of nonoperative intervention decreases with age, the success of surgical SCM release also decreases with age (Lee et al. 1986). A study of 80 patients looking at surgical outcomes in terms of radiographic cephalometry revealed that postsurgical changes in terms of craniofacial curvature and craniofacial asymmetry improvement were greater when the surgery was performed before the patient reached 5 years of age (Lee et al. 2012). Similarly Wirth et al. reported improvement in 75 % of patients aged <5 at the time of surgery compared to 40 % after the age of 10 using a bipolar tenotomy. Surgical treatment is recommended before the age of 5.
Preferred Treatment
For infants presenting with congenital muscular torticollis, the preferred approach (Fig. 17) is to initiate a physiotherapy program consisting of active stimulation and passive manual stretching exercises as well as counter-positioning. The technique is taught to the parents, and occasional visits to verify adequate technique and to monitor improvement are done with our outpatient physiotherapy program every 2–4 weeks. If there is inadequate improvement after 6 months of physiotherapy (i.e., residual head tilt and >15° of restricted movement in the lateral or rotational direction), botulinum toxin A administration can be considered as described by Joyce et al. (Joyce and de Chalain 2005). If there is still inadequate improvement, a distal release with Z-lengthening is performed around the age of 18–24 months followed by physiotherapy for 6 weeks. Patients are followed until skeletal maturity. For children presenting after 12 months of age, a trial of physiotherapy is warranted; however, if improvement is not noted within the first 3 months, one should proceed to botulinum toxin administration and/or distal surgical release with a Z-lengthening as necessary. For children presenting after 5 years of age, a bipolar release with distal Z-lengthening is performed followed by physiotherapy for 6 weeks. All patients should be followed for recurrence until skeletal maturity.
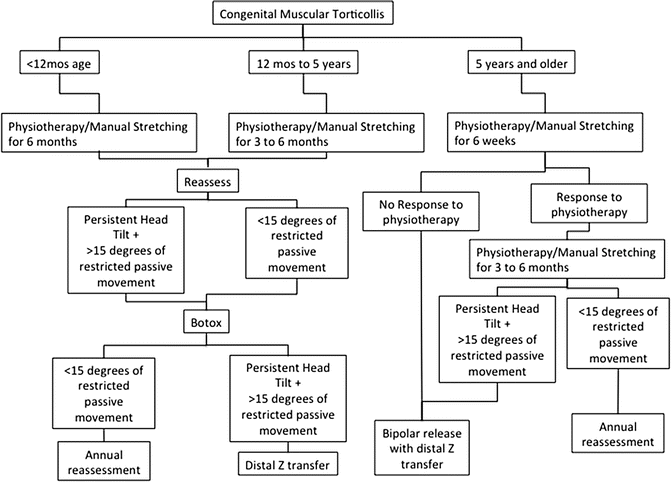

Fig. 17
Algorithm for the treatment of congenital muscular torticollis
Surgical Pitfalls and Prevention
Recurrence can be a problem, and although it occurs in less than 5 % of cases, it must be minimized. Early surgery has been related with recurrence and is thought to be secondary to scar formation that creates a tether. Surgery prior to age 1 is typically not recommended in order to minimize this problem. Inadequate release can also be the cause of recurrence as well as the cause of inadequate correction. In many cases fascial bands can tether the SCM and prevent an optimal release. It is important to place the SCM on stretch during the surgery and to palpate for any residual tight bands that should be released (under direct visualization). Also, the clavicular head fans out onto the clavicle, and often times the lateral aspect of the tendon is not adequately released at the outset. Careful dissection to locate the lateral edge of this tendon while the SCM is on stretch can minimize an inadequate release. In cases of recurrence after a distal release, a proximal release can be performed. In cases of recurrence after a bipolar release, a revision of the release can be performed; however, consultation with a head and neck surgeon is recommended to avoid complications with respect to spinal accessory nerve, facial nerve, and carotid sheath injury. Furthermore, recurrence may warrant a more detailed examination of the child as it may be a sign of a nonmuscular cause of torticollis, connective tissue disorder, or arthrogryposis.
The distal incision can be associated with an unsightly scar. This occurs when the incision is placed over the bony aspects of the sternoclavicular joint. With the head positioned to enable visualization of the SCM, the skin overlying this region is pulled superiorly due to extension of the head and neck, and therefore the skin incision is often inadvertently lower than expected. To avoid this problem, the distal skin incision should be placed 1 fingerbreadth above the clavicle. Finally, injury to the vessels and nerves is extremely uncommon although they are at risk when performing a proximal release. A careful examination of the facial nerve and spinal accessory nerve should be performed postoperatively, and should a deficit be noted, the patient should be referred to the appropriate service. The facial nerve is anterior to the SCM, and injury can be avoided by keeping the incision centered over the SCM posterior to the ear. Injury to the spinal accessory nerve can be minimized by careful dissection around the muscle before releasing it and performing the release in layers. A Penrose drain can be placed around the muscle to help bring it up out of the wound to improve visualization of the fibers, and the transection should be done under direct vision.
Congenital muscular torticollis | |
|---|---|
Potential pitfalls and preventions
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|