Topical Medications
Victoria Tutag Lehr
Mirjana Lulic Botica
Merene Mathew
Topical administration represents an important method of delivery of medications for infants and children. Clinicians must remember to inquire about use of topical medications or transdermal or “patch” delivery systems during a medication history. Patients and parents frequently overlook mentioning the use of topical medications during clinical examinations. Topically applied medications may undergo percutaneous absorption, resulting in systemic effects (1,2,3).
Developmental changes affecting the skin throughout infancy and childhood influence the rate and extent of absorption, metabolism, and bioavailability of topically administered medications (1). The skin is a major body organ for the newborn. Skin accounts for up to 13% of an infant’s total body weight compared with only 3% of an average adult’s body weight (2). This greater total body surface area ratio to body mass results in a much greater proportion of drug absorbed per kilogram of body weight for infants compared with adults (1,2,3,4,5). Infants are at increased risk for development of toxic drug serum concentrations with topical administration of medications, such as topical anesthetics, corticosteroids, antihistamines, and antiseptics (2,3,4,5).
Human skin is composed of two morphologically distinct layers (epithelial and mesenchymal) which originate from two different germ layers during development (2,3). Epithelial structures derived from the ectoderm are the epidermis, pilosebaceous–apocrine unit, eccrine unit, and nails. The ectoderm also generates hair and teeth. The mesoderm generates the mesenchymal structures—collagen, reticular and elastic fibers, blood vessels, muscles, and fat. These form the three layers of human skin: the epidermis, the dermis, and the subcutaneous tissue, which influence the absorption and metabolism of topically administered medications (4).
Epidermal development is markedly influenced by gestation (5,6). Before 30 weeks of gestation, the epidermis is thin, has few cell layers, and a poorly formed stratum corneum (6). This functional superficial layer acts as a barrier, composed of closely packed dead cells undergoing constant exfoliation. Maturation of the epidermis occurs at around 34 weeks of gestation. There is a profound postnatal effect on epidermal development in preterm infants, so histologically the epidermis of the most immature infant resembles that of a term infant by 2 weeks of age. These histological changes parallel development of barrier properties of newborn skin (4,5). Preterm infants younger than 35 weeks of gestation have less well-developed stratum corneum and thinner skin compared with older children and adults. A significant difference in thickness between the stratum corneum of term infants, children, or adults has not been demonstrated (6).
The dermis is beneath the epidermis and contains a rich supply of vascular beds, connective tissue, and lymphatics (6,7). At birth, skin is richly supplied by a disorderly horizontal, capillary network that organizes into the adult papillary loop pattern during the first 2 weeks of life (7). Skin cooling encourages maturation of capillary network loops (7).
Fatty connective tissue is the major component of the subcutaneous layer and starts accumulating around week 14 of gestation (2,3,4). Fat serves as insulation, cushioning, and an energy source. Fat storage continues to accumulate until birth. The dermis and subcutaneous layer contain sebaceous glands and sweat glands. Sebaceous glands are not fully functional until puberty, while the sweat glands will mature at day 5 of life (3,4).
Percutaneous absorption of a drug requires transfer from skin surface through the stratum corneum to the underlying epidermis and dermis (8,9,10). Factors contributing to percutaneous absorption of topical medications include physiochemical properties of the drug, concentration of drug in the vehicle, chemical and physical properties of the vehicle, thickness and hydration of the stratum corneum and epidermis, occlusion, and presence of inflamed, diseased, or damaged skin (11).
Passage through the stratum corneum is rate limiting for percutaneous absorption of an exogenous substance (11,12). The stratum corneum is composed of keratinized corneocytes surrounded by a lipid matrix, providing a barrier to percutaneous absorption. Major steps in percutaneous absorption include concentration gradient, the release of the drug from the vehicle into the skin (partition coefficient), and diffusion of the drug through the epidermis (diffusion coefficient) (10,11,12). The thicker stratum corneum on the palms and soles decreases absorption, whereas thinner skin of the eyelids, face, axillae, and genitals allows for increased absorption.
Percutaneous absorption of topically administered medications in infants differs from that in adults in several clinically significant aspects. Preterm infants have greater cutaneous perfusion and epidermal hydration compared with older infants, children, and adults (2,3,4,5). This may result in enhanced percutaneous absorption of topically applied medications, predisposing to systemic drug toxicity (2,3,4,5,13).
Hydration enhances permeability of hydrophilic drugs by increasing the diffusion constant (10,11,12). Although skin thickness is similar in infants and adults, infants have a greater degree of skin hydration and perfusion compared with adults, enhancing skin permeability (1,2,3,4,5). Hydration of the stratum corneum is greatest extent in the axillae, genitals including the diaper area, and the antecubital and popliteal fossae.
Molecular weight and size of drug significantly determine percutaneous absorption (12,13,14,15). Solubility of drug in the vehicle and tissue is also integral to drug absorption. In general, the more lipophilic the molecule, the more readily it penetrates the skin (15). The stratum corneum determines the rate of diffusion into the epidermis. The drug may diffuse down along a concentration gradient, bind to sites in the tissue, and undergo vasculature resorption or metabolism by a variety of mixed function monooxygenases or other enzymes (11).
Occlusion with plastic wrap during application of a topical medication will result in increased percutaneous absorption, predisposing to development of toxic serum concentrations of active ingredients or incipients. Occlusive dressings must be used with caution on infants and young children to avoid accumulating toxic serum drug concentrations from enhanced absorption (3).
Infected, broken, or abraded skin allows increased absorption of topical medications. Caution must be taken when applying topical medications to these areas on young infants and children for a prolonged duration of therapy. Monitoring parameters for potential toxicity will ensure safe use of topical medications on skin with an altered barrier.
Type of vehicle affects percutaneous absorption and patient compliance. A review of vehicle pharmacokinetics are discussed in further detail elsewhere (10,11). An ointment is composed of a lipophilic drug in a base such as petrolatum, mineral oil, waxes, or organic alcohols (9,10,13). Ointments impart a relatively high partition coefficient—defined as the relative solubility of a drug in the stratum corneum and vehicle—making them the most efficient vehicle for topical drug delivery (12). Emulsions are mixtures of two immiscible substances. Creams are classified as emulsions of oil in water or water in oil, depending on whether they can be washed off with water or not (9,10,13). An oil-in-water emulsion is more cosmetically acceptable, whereas a water-in-oil emulsion is more occlusive. Foams, emulsions of liquid and gas, may be easily applied to hair-bearing areas. Liquid preparations are divided into monophasic solutions, emulsions, and suspensions. Monophasic solutions include lotions, gels, and oils.
Parents and caregivers require appropriate instructions for safe and effective application of topical creams and ointments. Include a description of the area of application, as well as frequency and duration of use. Indicate whether the product should be rubbed into the skin or applied in a layer of specific thickness. Application of topical medications to large areas of skin of a young infant or child must be avoided secondary to increased skin to body weight ratio, predisposing to increased plasma concentrations of drugs. Caregivers should be instructed to wear a sterile, disposable glove when applying creams or ointments to broken skin. Topical medications should not be shared between patients to avoid cross-contamination.
The site of application should not be covered with an occlusive dressing unless increased absorption is desired. In addition, plastic film occlusive dressings have been associated with bacterial infection in preterm infants (2). Cautions about exposure to sunlight may be appropriate, as phototoxic reactions are possible with a variety of topical medications (16,17).
Topical Corticosteroids
Topical corticosteroids have been available since the 1950s and are the cornerstone therapy for inflammatory dermatoses, such as psoriasis, eczema, seborrheic, allergic or atopic dermatitis, insect bites, poison ivy, and other skin irritations (18,19,20). Large numbers of topical steroids are available in cream, ointment, lotion, gel, solution, and shampoo forms (18,21). Steroid preparations are grouped according to relative anti-inflammatory activity and agents in each group are approximately equivalent (Table 26.1) (18,21). The relative potency of a particular steroid product depends on the characteristics and concentration of the drug and vehicle. In general, ointments and gels are more potent than creams or lotions. However, some products have been formulated to yield comparable potency (22,23).
Mechanism of Action
Corticosteroids primarily act by binding to cytoplasmic glucocorticoid receptors in the cytosol, followed by translocation of the ligand–receptor complex that enters the nucleus to regulate gene transcription and the inflammatory process (24,25). Other actions of corticosteroids include immunosuppressive, antiproliferative, and vasoconstrictive effects (25,26). Topical corticosteroids are absorbed into skin cells inhibiting inflammatory cells via actions on mediator release and function, inflammatory cell function and release of lysosomal enzymes. The cascade of inflammatory cytokines such as prostaglandins and other inflammatory substances is blocked from release as skin reacts to allergens or irritants, ultimately reducing inflammation and relieving symptoms of pruritus (24,25,26).
Indications and Clinical Use
Common conditions responsive to topical corticosteroids include atopic and seborrheic dermatitis; contact, diaper, and irritant dermatitis; neurodermatitis; dyshidrotic eczema; lichen planus; lichen simplex chronicus; nummular dermatitis; pityriasis rosea; psoriasis; and inflammatory phase of xerosis (20,21).
Table 26.1 Corticosteroid Preparations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pharmacokinetics
Pharmacokinetics of topical corticosteroid use is determined by potency of the corticosteroid, its vehicle, and the skin onto which it is applied (26). Corticosteroid potency is determined in part by its chemical structure and by manipulation of the steroid molecule to produce compounds with greater lipophilicity, fewer mineralocorticoid properties, and higher potency. Corticosteroids can be divided into two classes: fluorinated and nonfluorinated (26). Fluorinated steroids refer to those steroids that have been chemically altered to increase their potency. For example, halogenation at any position increases potency of the steroid. This modification also increases mineralocorticoid effects, enhancing systemic side effects. Other
chemical modifications include hydroxylation, addition of double bonds, alteration of functional groups (esterification and methylation), and addition of ketone groups. The altered structure and resulting increase in potency may be due to increased lipophilicity, percutaneous absorption, and/or glucocorticoid receptor binding activity (23,24,25,26). The vehicle indirectly affects topical corticosteroid potency by influencing the environment in which the corticosteroid is absorbed. For example, through occlusion, ointments help hydrate the stratum corneum, thereby enhancing penetration of corticosteroids (26,27,28,29,30). In addition, solvents such as propylene glycol and ethanol affect solubility of corticosteroids, enhancing percutaneous absorption. Finally, thickness and integrity of the stratum corneum are inversely proportional to absorption of topical corticosteroids (28,29). Penetration into eyelid skin is therefore better than into palmar skin, and inflamed or diseased skin is more readily penetrated than intact skin (28,31).
chemical modifications include hydroxylation, addition of double bonds, alteration of functional groups (esterification and methylation), and addition of ketone groups. The altered structure and resulting increase in potency may be due to increased lipophilicity, percutaneous absorption, and/or glucocorticoid receptor binding activity (23,24,25,26). The vehicle indirectly affects topical corticosteroid potency by influencing the environment in which the corticosteroid is absorbed. For example, through occlusion, ointments help hydrate the stratum corneum, thereby enhancing penetration of corticosteroids (26,27,28,29,30). In addition, solvents such as propylene glycol and ethanol affect solubility of corticosteroids, enhancing percutaneous absorption. Finally, thickness and integrity of the stratum corneum are inversely proportional to absorption of topical corticosteroids (28,29). Penetration into eyelid skin is therefore better than into palmar skin, and inflamed or diseased skin is more readily penetrated than intact skin (28,31).
Therapeutic Guidelines for Corticosteroid Treatment
The risk of adrenal suppression with prolonged or overuse can be decreased by judicious prescribing of initial quantities and limiting number of additional refills.
Selection of the specific corticosteroid strength and vehicle depends on location, extent of the skin condition, patient’s age, and anticipated duration of treatment.
The extent of absorption is based not only on drug potency but rather on the vehicle in which it is formulated. Ointment bases enhance penetration of the drug and may be preferred for thick, lichenified lesions. Creams are preferred for acute and subacute dermatoses. Solutions, lotions, oils, gels, or foams should be prescribed for hair-bearing areas where a non–oil-based vehicle is required.
Corticosteroid potency must be considered when prescribing these agents. In general, low-to-medium potency agents should be utilized for treating acute thin inflammatory lesions, whereas, very high potency and high-potency corticosteroids should be reserved for treating chronic hyperkeratotic, lichenified lesions.
Low-potency corticosteroids should be used on areas with a thin stratum corneum such as the face, skin folds, and diaper area. Low-potency corticosteroids are preferred for infants and especially low-birth-weight infants who have immature skin with poorly formed epidermal barrier leading to accidental poisoning from percutaneous absorption of chemicals and superficial damage from use of adhesives.
Higher-potency corticosteroids may be used if necessary, but for a limited duration and should be reserved for areas with a thick stratum corneum such as the palms and soles of feet. Very potent corticosteroids should not routinely be used for longer than 2 to 3 weeks.
Tapering from a more potent to a less potent topical corticosteroid will help prevent a rebound flare. Avoid abrupt discontinuation.
Topical corticosteroids should not be used on ulcerated or atrophic skin.
Corticosteroid–antifungal combinations should be avoided for treatment of dermatophytoses.
Laboratory tests for adrenal suppression should be performed following prolonged treatment particularly over large areas of skin.
Adverse Effects
The most common side effects of topical corticosteroids are local: atrophy and striae, both of which may be irreversible (19,20). Other cutaneous side effects include hypopigmentation, telangiectasias, purpura, tinea and scabies incognito, granuloma gluteale infantum, acneiform eruptions, perioral dermatitis, and steroid rosacea. These side effects are more commonly seen with inappropriate use of high-potency fluorinated topical corticosteroids applied for prolonged periods of time on the face and intertriginous areas. Applied periocularly, there is a risk for development of glaucoma or cataracts. Allergic contact dermatitis to corticosteroids should be considered if a patient’s condition is unresponsive or made worse with treatment (19,27). Use of combination corticosteroid–antifungal preparations for treatment of dermatophytoses is associated with persistent and recurrent infections (29,30). Tachyphylaxis—diminished response to prolonged application—may develop with prolonged use of topical steroids (22).
The more serious and dreaded complications of topical corticosteroid use are systemic side effects (19). These side effects have been associated with short-term use of high-potency topical steroids and with even short-term use of low-potency formulations. Such effects are identical to those of systemically administered corticosteroids and include suppression of the hypothalamic–pituitary–adrenal axis (HPA), Cushing syndrome, failure to thrive, poor linear growth, hyperglycemia, and glycosuria. Factors augmenting systemic absorption include application of more potent steroids, use over large surface areas, application on skin folds, prolonged use, occlusive dressings, younger age (infants and young children), and liver or renal disease (26,32).
Monitoring Parameters for Patients
Parents and caregivers of children and infants initiated on topical corticosteroid therapy should be educated about potential side effects (19,28). Burning, stinging, itching, and redness occur in the affected area with initial application, but dissipate over time as the body adjusts to the medication is a common concern. Patients or caregivers should closely monitor for skin thinning or discoloration. Topical corticosteroids should not be used near the eyes, especially in patients with a history of glaucoma. Treatment durations beyond 2 to 3 weeks for very potent topical corticosteroids should be discouraged. The child’s health care provider should be consulted immediately if any vision problems, persistent headaches, increased thirst or urination, unusual weakness or weight loss, and dizziness occur, as these symptoms may indicate HPA suppression from systemic steroid absorption (18,21,24).
Topical Immunomodulators
Topical corticosteroids have been the mainstay of therapy for atopic dermatitis, but side effects with higher doses and prolonged application of steroids have limited their use, especially in infants and children (19,33). Atopic dermatitis is a commonly encountered eczematous skin condition presenting in children of all ages. Although the exact etiology of atopic dermatitis is unknown, it may involve genetic and environmental factors as well as defects in skin barrier and immune function (9,33). Among children in whom atopic dermatitis is diagnosed during first 2 years of life, about half develop asthma.
When atopic dermatitis does not improve with conservative measures such as generous use of emollients, alternative therapies should be considered (33,34,35). Short courses of topical corticosteroids have been traditionally treated disease flares, yet are limited by side effect potential (19,20,33). In the late 1980s, a topical formulation of cyclosporine was developed as an alternative treatment for atopic dermatitis without the corticosteroid side effect profile. However, cyclosporine was found to be ineffective due to large molecular size and poor dermal penetration.
Topical calcineurin inhibitors (TCIs), tacrolimus (Protopic®) and pimecrolimus (Elidel®), are much smaller molecules than cyclosporine and better able to penetrate the skin causing local immunosuppression (33,34,35). These agents are currently FDA approved for management of atopic dermatitis in patients 2 years of age and older (36,37,38,39).
Tacrolimus and Pimecrolimus (Topical Calcineurin Inhibitors)
Tacrolimus is a macrolide immunosuppressant isolated from the fungus Streptomyces tsukubaensis in 1984 (34,35). Pimecrolimus, also a macrolide immunosuppressant, is derived from ascomycin, a natural product of Streptomyces hygroscopicus var. ascomyceticus (35).
Mechanism of Action
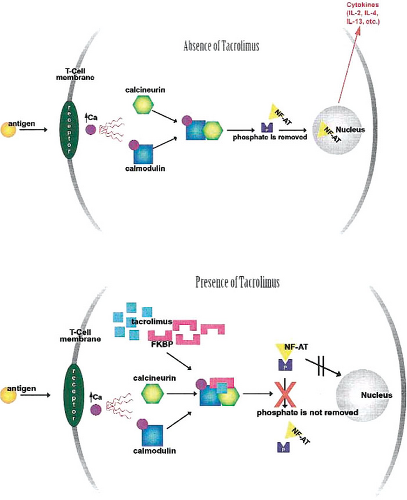
Although the mechanism of action of tacrolimus in atopic dermatitis is not completely understood, in vitro studies reveal that topical tacrolimus binds to specific T-cell receptors resulting in the inhibition of T-lymphocyte activation (Fig. 26.1) (34,40,41). Tacrolimus then forms a complex
with FKBP-12, calcium, calmodulin, and calcineurin resulting in the inactivation of the phosphatase activity of calcineurin (34). This results in the prevention of dephosphorylation and translocation of the nuclear factor of activated T cells (NF-AT). NF-AT is a nuclear component that is potentially responsible for initiation of gene transcription for lymphokines such as interleukin 2 (IL-2) and interferon gamma (IFN-#979;). Thus, these series of reactions inhibit the transcription of the genes involved in lymphokine formation (34). Tacrolimus also inhibits transcription of genes that encode for the markers involved in the early stages of T-cell activation such as IL-3, IL-4, IL-5, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor α. Release of preformed mediators from skin mast cells and basophils has also been shown to be inhibited by tacrolimus (34,41).
with FKBP-12, calcium, calmodulin, and calcineurin resulting in the inactivation of the phosphatase activity of calcineurin (34). This results in the prevention of dephosphorylation and translocation of the nuclear factor of activated T cells (NF-AT). NF-AT is a nuclear component that is potentially responsible for initiation of gene transcription for lymphokines such as interleukin 2 (IL-2) and interferon gamma (IFN-#979;). Thus, these series of reactions inhibit the transcription of the genes involved in lymphokine formation (34). Tacrolimus also inhibits transcription of genes that encode for the markers involved in the early stages of T-cell activation such as IL-3, IL-4, IL-5, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor α. Release of preformed mediators from skin mast cells and basophils has also been shown to be inhibited by tacrolimus (34,41).
Pimecrolimus has a mechanism of action similar to tacrolimus (35). The drug binds with increased affinity to macrophilin-12 (FKBP-12) and inhibits T-cell activation by preventing transcription of early cytokines such as IL-2 and IFN-#979; (Th1-type) and IL-4 and IL-10 (Th2-type) synthesis from T cells. Pimecrolimus also prevents release of inflammatory cytokines and mediators from mast cells poststimulation with antigen/immunoglobulin E complex in vitro (40,41). Studies demonstrate that pimecrolimus, unlike corticosteroids, interferes with the inflammatory cascade with no effect on keratinocytes, fibroblasts, endothelial cells, langerhans cells, the hypothalamus, or adrenal gland (35).
Indications and Clinical Use
Topical use of tacrolimus and pimecrolimus is FDA approved as second-line therapy for moderate-to-severe atopic dermatitis in children older than 2 years (39). Off-label use of both tacrolimus and pimecrolimus for first-line therapy has been rapidly increasing (36,40,41,42,43). This may be due to a perception by clinicians of a better safety profile compared with topical corticosteroids (40). Between June 2003 and May 2004, there were approximately 2 million prescriptions for tacrolimus and pimecrolimus given for children in the United States with approximately half a million of these for children younger than 2 years (40). In reality, long-term safety of these agents is unknown.
Based on concerns of the FDA regarding the increased risk of malignancy associated with systemic immunosuppression with high dose and prolonged therapy with oral calcineurin inhibitors (cyclosporine and tacrolimus), a black-box warning is included in the labeling for use of these agents. This is based on the theoretical risk calculated from animal studies after oral dosing, data from oral use in transplant patients, and rare reported cases of malignancies (39).
Efficacy of tacrolimus was using a randomized, double-blinded, vehicle-controlled study in children 7 to 16 years of age, with 0.03%, 0.1%, and 0.3% tacrolimus ointment prescribed twice-daily treatment compared with vehicle therapy for 23 days (37). Clinical assessment revealed that 67% to 70% of subjects in the three treatment groups had 75% improvement in symptoms compared with 38% of subjects in the vehicle group (37). Furthermore, tacrolimus ointment was shown to be more effective in children with moderate-to-severe atopic dermatitis, with a faster onset of action and similar safety profile compared with pimecrolimus cream (38). Both TCIs have been to treat other inflammatory skin conditions such as psoriasis, lichen planus, seborrheic dermatitis, allergic contact dermatitis, and vitiligo (16,41).
Despite long-term and short-term studies showing efficacy and safety of topical TCIs, it is prudent to prescribe these medications with caution using recommended guidelines (39). When prescribing topical tacrolimus and pimecrolimus, FDA urges health care providers to consider the following: (a) Prescribe only as second line for short-term or intermittent long-term treatment for atopic dermatitis in patients who are not responsive or intolerant to conservative measures and conventional therapies; (b) The effect of these immunomodulator agents on a developing immune system is not known; therefore, avoid their use in children younger than 2 years; (c) Long-term safety profile (>1 year duration of treatment) is unknown, so limit the use of these therapies to short noncontinuous periods of treatment time; (d) Children and adults with compromised immune system should not be prescribed these agents; and (e) Use the minimum amount required to alleviate patient’s symptoms (36,39).
TCIs are beneficial for maintenance therapy of atopic dermatitis, once acute control of skin exacerbation is established with topical steroids (40). An open-label study examined combining TCIs with intermittent topical steroids demonstrated greater benefit in long-term atopic dermatitis lesions with combination therapy than with steroids alone (40,43). Tacrolimus and pimecrolimus topical therapy has demonstrated efficacy in management of head and neck dermatitis where steroid use is limited (43).
Pimecrolimus has been used off label for dermatitis in infants (42). During a 6-week double-blind, vehicle-controlled study of 186 infants (3 to 23 months) with mild-to-moderate atopic dermatitis, twice-daily application of pimecrolimus was effective in clearing 54.5% of patients and resulted in improvement in 70% of patients (42). Treatments used in this study included moderately potent topical corticosteroids during flares, pimecrolimus at the first sign of symptoms, and emollients alone for disease-free intervals.
Pharmacokinetics
Tacrolimus, when ingested orally, undergoes hepatic metabolism; however, with topical application, very little drug is systemically absorbed (33,41). Pimecrolimus also does not show any evidence of dermal-mediated drug metabolism (35). In 80% of samples in a study of patients using tacrolimus for the treatment of moderate-to-severe atopic dermatitis, tacrolimus serum concentration was below the detectable concentration (37).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree