Basic schemes of coagulant and anticoagulant pathways are shown in Figures 25-1 and 25-2. Many of the heritable thrombophilias involve the protein C pathway. They typically involve a deficiency, abnormality, or both in anticoagulant proteins, leading to a procoagulant state. However, some thrombophilias, such as the prothrombin G20210A mutation lead to an increase in procoagulant proteins, also increasing the risk for thrombosis. Most thrombophilias are considered to be hereditary. These are usually associated with genetic mutations leading to the abnormalities in anticoagulant or procoagulant proteins. Others are acquired over time, often due to the onset or flare of an autoimmune condition. The acquired thrombophilia that is most common and relevant to obstetricians is an antiphospholipid syndrome (APS). A considerable proportion of thromboses associated with pregnancy occur in women with thrombophilias. Consequently, it is critical for obstetric providers to become familiar with them so as to optimally treat and prevent thromboembolism during pregnancy in women with thrombophilias.
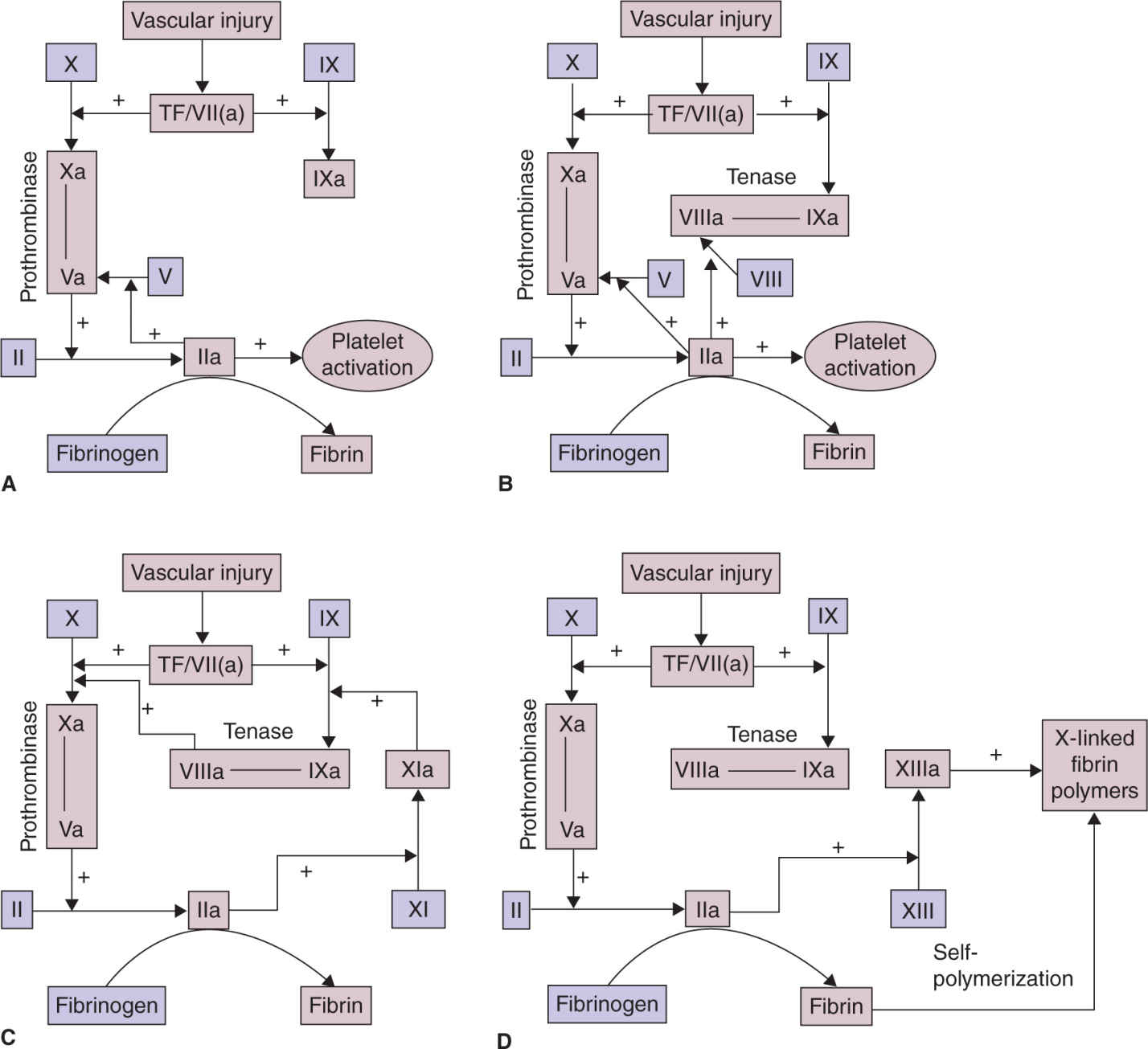
FIGURE 25-1. Fibrin plug formation. A, After vascular disruption, factor VII (a) binds to tissue factor (TF) to form the TF/VIIa complex, which activates factor X and factor IX. Factor Xa binds to factor Va, which has been activated by thrombin (factor IIa) or released from platelet α-granules. The Xa/Va complex catalyzes the conversion of prothrombin (factor II) to thrombin factor (factor IIa), which converts fibrinogen to fibrin and activates platelets. B, The clotting cascade is amplified by clotting reactions that occur on adjacent activated platelets. Locally generated factor IXa binds to factor VIIIa, which is activated by thrombin. The factor IXa/VIIIa complex then generates factor Xa. C, Coagulation is further boosted by the thrombin-mediated activation of factor XI to factor XIa, which also activates factor IX. D, The stable hemostatic plug is formed when fibrin monomers self-polymerize and are cross-linked by thrombin-activated factor XIIIa. Reproduced with permission from Creasy RK, Resnick R, Iams J, et al (eds): Creasy Resnick’s Maternal-Fetal Medicine: Principles and Practice. 7th ed. Philadelphia, PA: Saunders/Elsevier;2014.5
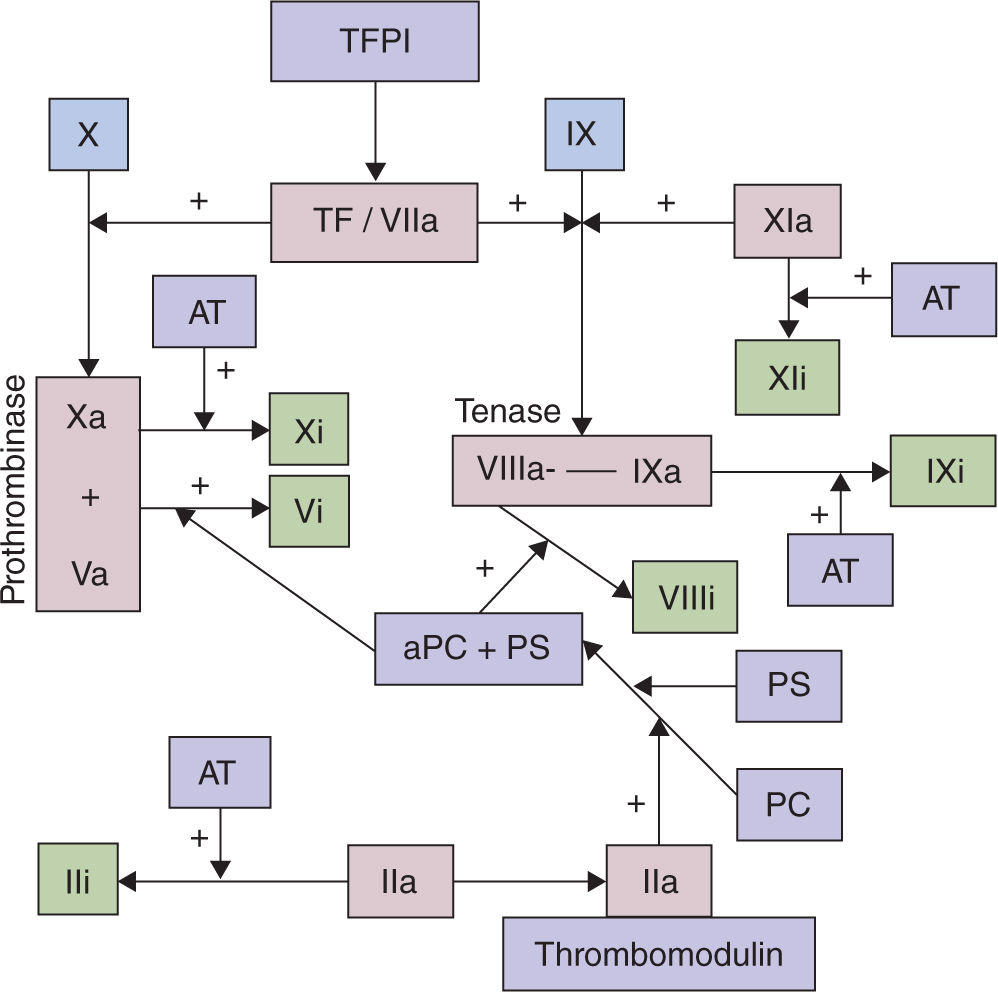
FIGURE 25-2. The anticoagulant system. Tissue factor pathway inhibitor (TFPI) binds with tissue factor (TF) and factor VIIa. Thrombin, after binding to thrombomodulin, can activate protein C (PC) when bound to the endothelial protein C receptor. Activated protein C (aPC) then binds to its cofactor, protein S (PS), to inactivate factors VIIIa and Va. Antithrombin (AT) potently inhibits factor Xa and thrombin. Reproduced with permission from Creasy RK, Resnick R, Iams J, et al (eds): Creasy Resnick’s Maternal-Fetal Medicine: Principles and Practice. 7th ed. Philadelphia, PA: Saunders/Elsevier;2014.5
It is also important to recognize that thrombophilias are common in healthy, asymptomatic individuals. Most do not develop VTE and would never know that they have a thrombophilia if they were not tested. The reasons for these mutations persisting in the population are uncertain. However, it has been speculated that they decrease the chance of hemorrhage, especially associated with childbirth. Indeed, there are some data to support this hypothesis.2 This certainly is an appealing evolutionary explanation for the high prevalence of these seemingly “unhealthy” mutations.
In addition to thrombosis, thrombophilias have been linked to an increased risk of adverse obstetric outcomes. Antiphospholipid antibodies have been known to be associated with fetal death and other obstetric complications for decades. The pathophysiology remains unclear but in part is thought to be due to thrombosis in the uteroplacental circulation, leading to placental thrombosis and infarction, and ultimately placental insufficiency. Outcomes in women with APS appear to be improved in the setting of anticoagulant therapy. Accordingly, it was a logical assumption that many recently identified heritable thrombophilias also are associated with adverse obstetric outcomes. Indeed, numerous case-control studies seemed to confirm this hypothesis. However, it has become clear that most women with thrombophilias have normal obstetric outcomes. Also, it is not clear that treatment with anticoagulant therapy decreases the chances of obstetric complications in women with heritable thrombophilias.
HERITABLE THROMBOPHILIAS AND THROMBOSIS
Factor V Leiden Mutation
The factor V Leiden mutation is a point mutation in the factor V gene that causes factor V to be “refractory” to inactivation by activated protein C. There are other causes of resistance to activated protein C, but the factor V Leiden mutation is the most common. It is inherited in an autosomal dominant fashion and heterozygotes are affected. The prevalence ranges between 1% and 15% in the general population depending upon race/ethnicity. It is present in about 5% of Europeans, but is quite rare in African blacks and most Asians.3,4 It is present in about 3% of African Americans.4 Homozygotes are less common and account for less than 1% of the general population.
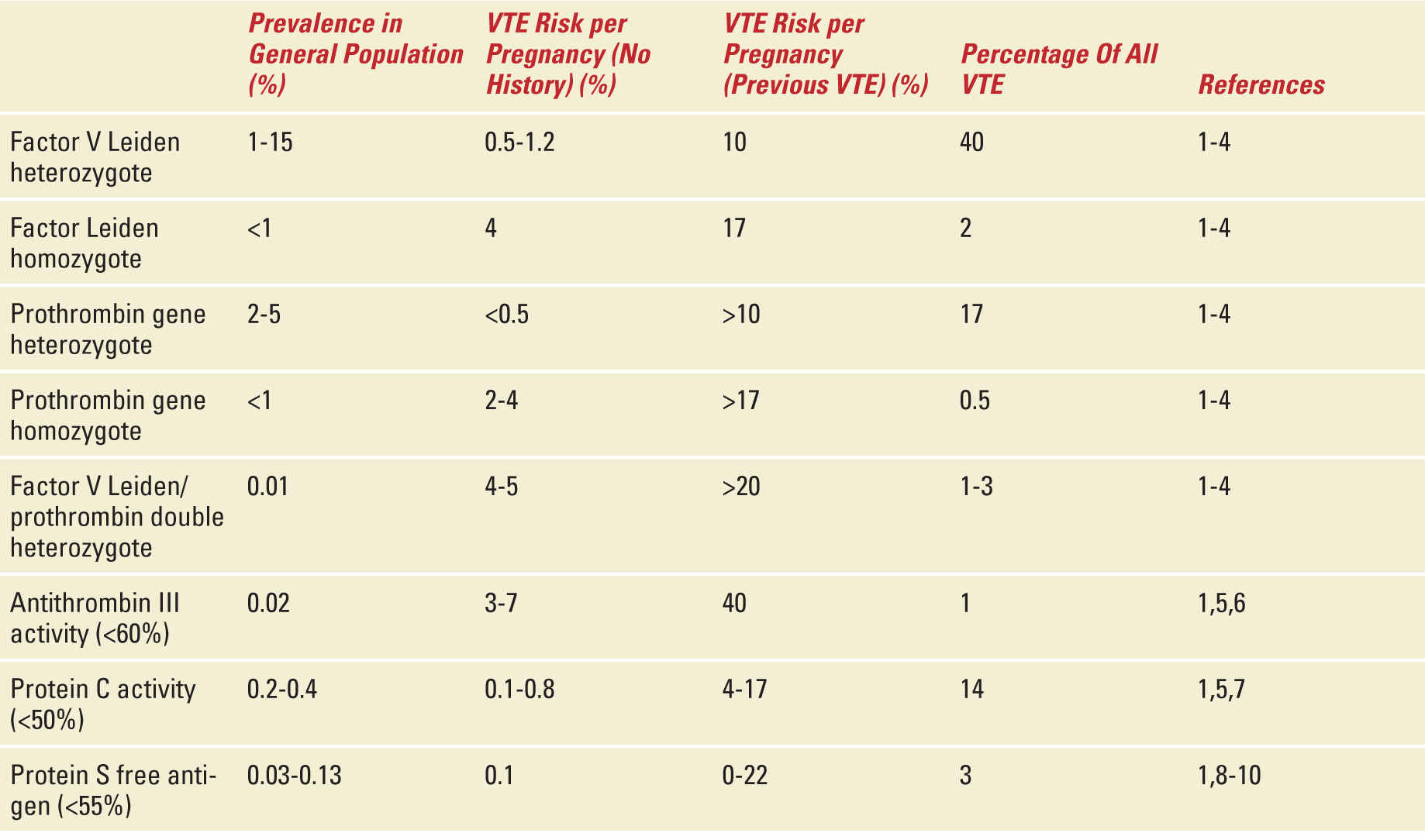
Heterozygous individuals are at about a fivefold increased risk of VTE and a lifetime risk of 35%.5 Moreover, factor V Leiden is associated with approximately 40% of VTE in pregnancy or the puerperium. However, the prospective risk in pregnancy in women with no personal history or family history of VTE is relatively low and is estimated as 5 to 12 per 1,000.6–8 The risk increases to about 10% if the woman has a personal history of VTE.4,8 The risk of thrombosis in pregnancy is about 1% to 2% for homozygotes with no history of VTE and about 17% for those with a prior VTE.4,8 The epidemiology of the factor V Leiden mutation and other thrombophilias is shown in Table 25-2.
TABLE 25-2 | Risk of Venous Thromboembolism With Different Thrombophilias |

Prothrombin G20210A Mutation
This point mutation results in increased translation of the prothrombin gene, leading to an increase in circulating prothrombin. It occurs in about 3% of Europeans. Although it is associated with 17% of VTE in pregnancy, heterozygous women with no personal history of VTE have a less than 1% risk of VTE in pregnancy.6–8 This increases only slightly with a family history of VTE but goes up to at least 10% in women with prior VTE.4,8 As expected, the risk is higher in women who have two copies of the mutation. Homozygotes for the prothrombin G20210A mutation and no prior VTE or family history of VTE have a 2% to 4% risk of VTE in pregnancy.4,8 The risk increases to over 17% if homozygous women have had a prior VTE.
Some individuals are heterozygous for both the prothrombin G20210A and factor V Leiden mutations. This is termed “compound heterozygosity.” The condition is rare, affecting about 1 in 10,000 women.6,7 They are at substantially greater risk for thrombosis than women with either mutation alone. Even if they have no personal or family history of VTE, their risk of VTE in pregnancy is 4% to 5%.4,8 This risk increases to over 20% if they have a personal history of prior VTE.4,8 Other thrombophilias also may occur in combination, likely resulting in enhanced coagulability. However, there are no precise estimates of risk during pregnancy owing to a lack of data.
Protein C Deficiency
Deficiencies of protein C can result from over 160 mutations. Each produces a slightly different phenotype.3 The condition is detected by measuring protein C antigen and activity using a functional assay. Type I disease involves a reduction in both antigen and activity, whereas type 2 is characterized by normal levels of antigen but decreased activity. Protein C deficiency is uncommon, affecting about 0.2% to 0.3% of individuals.4 The prevalence is affected by the assays and thresholds used to define the condition. The risk of VTE in pregnancy in women with protein C deficiency and no personal history of VTE is estimated as 0.1% to 0.8%.4 The risk in women with a personal or family history of VTE is 2% to 7%.4 Homozygous protein C deficiency is quite rare. Affected neonates develop a condition termed purpura fulminans and they require life-long anticoagulation.9
Protein S Deficiency
As with protein C, protein S deficiency results from over 100 mutations.3 Testing involves assessment of both the protein S antigen and functional assays and several subtypes and phenotypes exist. The risk of thrombosis during pregnancy is estimated to be 0.1 % in women with no personal or family history of VTE.4,8 However, the risk is estimated to be 6% to 7% in those with a family history of VTE and up to 22% in those with a personal history of VTE.4,8 Neonates who are homozygous for mutations causing protein S deficiency also develop purpura fulminans.9
Antithrombin III Deficiency
Antithrombin III (AT III) deficiency is one of the most thrombogenic thrombophilias, increasing the risk of VTE by 250-fold.3 It is uncommon, occurring in about 1 in 2500 individuals. It is caused by over 250 mutations and as with protein C and S, there may be deficiencies or abnormalities in the AT III protein, or both. The risk of thrombosis during pregnancy in women with no prior VTE is estimated as 3% to 7%. It rises to 40% in those with a prior VTE.4,8
Hyperhomocysteinemia
Elevated levels of homocysteine have been linked to an increased risk of VTE. In many cases, elevated levels are due to various mutations in the methionine metabolic pathway. The most common of these are homozygosity for the MTHFR C677T and A1298C mutations. It is noteworthy that it requires homozygosity for individuals to be affected. However, it has become clear that elevated levels of homocysteine are only a very small risk factor for VTE.10 In addition, homocysteine levels normally decrease in pregnancy and levels increase with folic acid supplementation, which routinely occurs during pregnancy. Thus, hyperhomocysteinemia is rare during pregnancy, even in the setting of individuals who are homozygous for these mutations. Also, vitamin supplementation in nonpregnant individuals with hyperhomocysteinemia does not reduce the risk of VTE.11 Finally, homozygosity for these mutations is common in Europeans (10% to 16% for MTHFR C677T and 4% to 6% for A1298C).12 Taken together, there is insufficient evidence to support testing (or treating) women for these mutations or hyperhomocysteinemia with or at risk of VTE in pregnancy.
Other Thrombophilias
Numerous other thrombophilias have been described and new ones are identified each year. Several other mutations in the factor V gene have been linked to some risk of VTE. None are common enough to warrant routine screening. Other mutations affect genes in the fibrinolytic pathway. The promoter region of the plasminogen activator inhibitor-1 (PAI-1) gene has two polymorphisms, A675 4G/5G and A844G, which lead to increased levels of PAI-1. They may increase the risk of VTE in women with other thrombophilias but are not meaningful risk factors for VTE alone. Several other thrombophilias have been described including protein Z deficiency, polymorphisms in the fibrinogen beta chain, apolipoprotein B, factor XIII, and tissue factor pathway inhibitor genes. However, these all have uncertain relationships to VTE risk. Testing for all of these “other” thrombophilias is not currently recommended. Nonetheless, clinicians should be alert to new information as it becomes available because some of these (or as yet unidentified thrombophilias) may eventually prove to be clinically important.
HERITABLE THROMBOPHILIAS AND OBSTETRIC COMPLICATIONS
Heritable thrombophilias have been linked to adverse pregnancy outcomes in numerous case control studies. For example, factor V Leiden was associated with an OR of 2.01 (95% CI, 1.13-3.58) for early pregnancy loss (<13 weeks gestation) in a meta-analysis of 31 studies.13 The association was stronger for late (>19 weeks gestation) nonrecurrent fetal loss with an OR of 3,26 (95% CI, 1.82-5.83).13 The same meta-analysis noted an OR of 2.3 (95% CI, 1.2-4.79) for recurrent early pregnancy loss and 2.56 (95% CI, 1.04-6.29) for recurrent loss later in gestation.13 The association in the majority of studies for most thrombophilias has been stronger with pregnancy loss later in gestation than with early pregnancy loss.
In addition to pregnancy loss, thrombophilias have been implicated in obstetric complications caused by placental insufficiency. These include preeclampsia, fetal growth restriction (FGR), and abruption. As with pregnancy loss, an association between thrombophilias and these problems has been noted in case-control studies. However, data are mixed and the association between thrombophilias and these problems is less clear than with late pregnancy loss.5
In contrast to retrospective case-control studies, several large prospective cohort studies showed no association between thrombophilias and adverse obstetric outcomes. For example, 2480 Swedish women were assessed for factor V Leiden early in pregnancy, without clinician knowledge of the results.2 Those with factor V Leiden had an eightfold increase in the risk for VTE. However, they had no increase in the rate of early or late pregnancy loss, preeclampsia, FGR, or abruption.2 A similar study was performed in a multicenter US population conducted by the Eunice Kennedy Shriver Maternal Fetal Medicine Units Network (MFMU) in almost 5000 women.14 Women testing positive for the factor V Leiden mutation had no increase in the incidence of pregnancy loss, preeclampsia, FGR, abruption, or VTE.14 Another study using the same cohort found no association between the prothrombin G20210A mutation and any obstetric complication.15
In part, the different results between the case-control and prospective cohort studies are due to the inherent bias in retrospective studies, often conducted in small numbers of women. Also, it is clear that women with thrombophilias and prior adverse obstetric outcomes likely comprise a different population than women with thrombophilias and no adverse obstetric outcomes. Perhaps it requires a series of events or “two-hits” for women with thrombophilias to have an adverse obstetric event. Regardless, the key observation is that the vast majority of women with thrombophilias have normal pregnancy outcomes and should be reassured. At worst, thrombophilias should be viewed as minor “risk factor” for, rather than a “cause” of, obstetric complications.
Initial reports linking thrombophilias to adverse pregnancy outcomes generated considerable enthusiasm for screening women with obstetric complications for these conditions and treating positive women with anticoagulant therapy. Also, several case series and one small, randomized controlled trial reported improved outcomes in women with thrombophilias and prior pregnancy complications.16,17 Given the relatively high prevalence of some thrombophilias, led to large numbers of women being treated with anticoagulant therapy during pregnancy.
Unfortunately, it is not clear that treatment improves outcome in these women and the preponderance of evidence suggests that anticoagulant therapy does not reduce the risk of adverse obstetric outcomes in women with thrombophilias. A retrospective nonrandomized trial of women with thrombophilias and adverse obstetric outcomes showed no difference in subsequent outcomes in pregnancies that were and were not treated with anticoagulant therapy.18 A small prospective randomized trial compared prophylactic doses of Dalteparin plus low-dose aspirin to low-dose aspirin alone and women with thrombophilias. Live birth rates were excellent and similar (almost 80%) in both groups.19 Similarly, studies comparing anticoagulant therapy and placebo in women with recurrent pregnancy loss without APS showed no increase in live births with therapy.20,21 Finally, a recent, large, multicenter, open label, randomized trial of thromboprophylaxis with Dalteparin compared to no treatment, failed to show a benefit for thromboprophylaxis.22 This latter study included only women with thrombophilias and included almost 300 women.22
Taken together, these data suggest no utility for testing patients with obstetric complications for heritable thrombophilias. The conditions are common, most women have normal obstetric outcomes and treatment has not been proven to improve outcomes. Thrombophilia testing should be limited to women with or at risk for thromboembolism in the absence of more compelling data.4
TESTING FOR HERITABLE THROMBOPHILIAS
Testing for heritable thrombophilias is appropriate in most women with a personal history of thromboembolism. One could argue that testing is unnecessary in cases wherein the VTE is associated with a nonrecurrent risk factor such as orthopedic surgery. Nonetheless, there is enough evidence to justify testing all women with VTE who are pregnant or considering pregnancy. It is also recommended in women with a first degree relative (parent, sibling, child) with either thrombophilia or thromboembolism. As stated earlier, it is not currently advised for women with obstetric complications.
It is important to note that testing for thrombophilias may be influenced by pregnancy, anticoagulant therapy, and acute thrombosis. The factor V Leiden and prothrombin G20210A mutations are DNA-based tests that are not affected by pregnancy, anticoagulant therapy, or thrombosis. They are reliable at any time. In contrast, testing for AT III, protein C and protein S deficiencies are accomplished via clotting tests. As such, they are unreliable in the setting of anticoagulant therapy and acute thrombosis. In addition, protein C and especially protein S levels are normally reduced during pregnancy. Diagnosis of protein C deficiency is usually still reliable. However, substantially lower thresholds to define protein S deficiency (eg, less than 30% in the second and 24% in the third trimesters) should be used if testing is done during pregnancy.
There are insufficient data to justify testing women for hyperhomocysteinemia or associated mutations such as the MTHFR C677T or A1298C. The same is true for the other less common thrombophilias described earlier.
TREATMENT OF HERITABLE THROMBOPHILIAS IN PREGNANCY
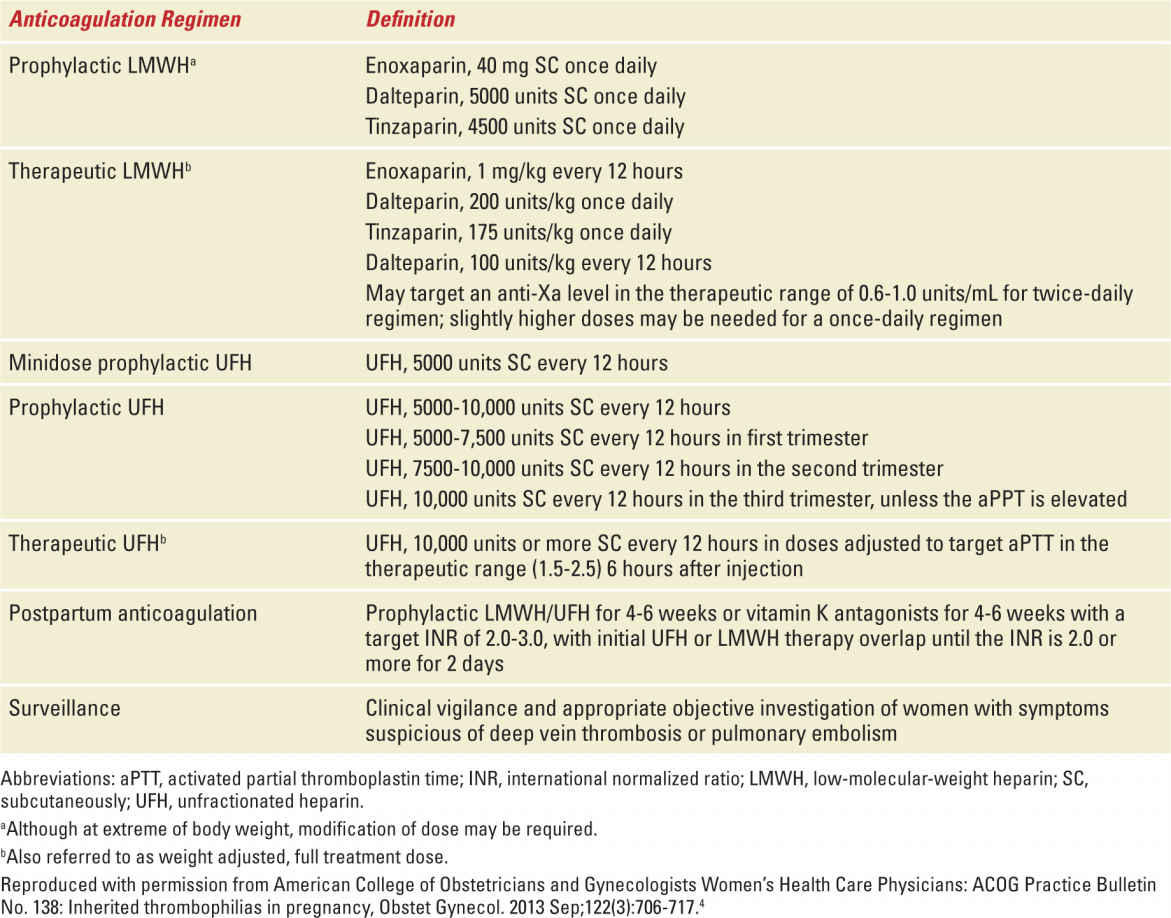
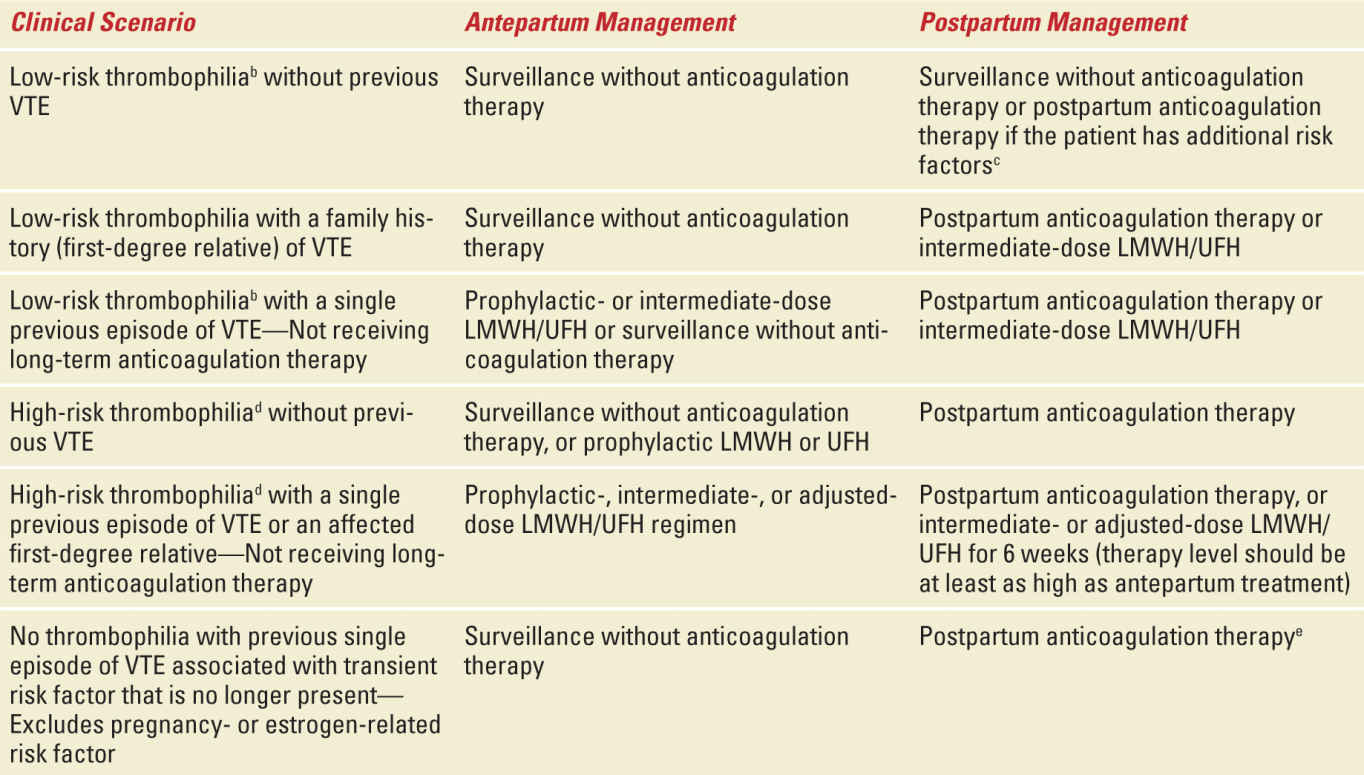
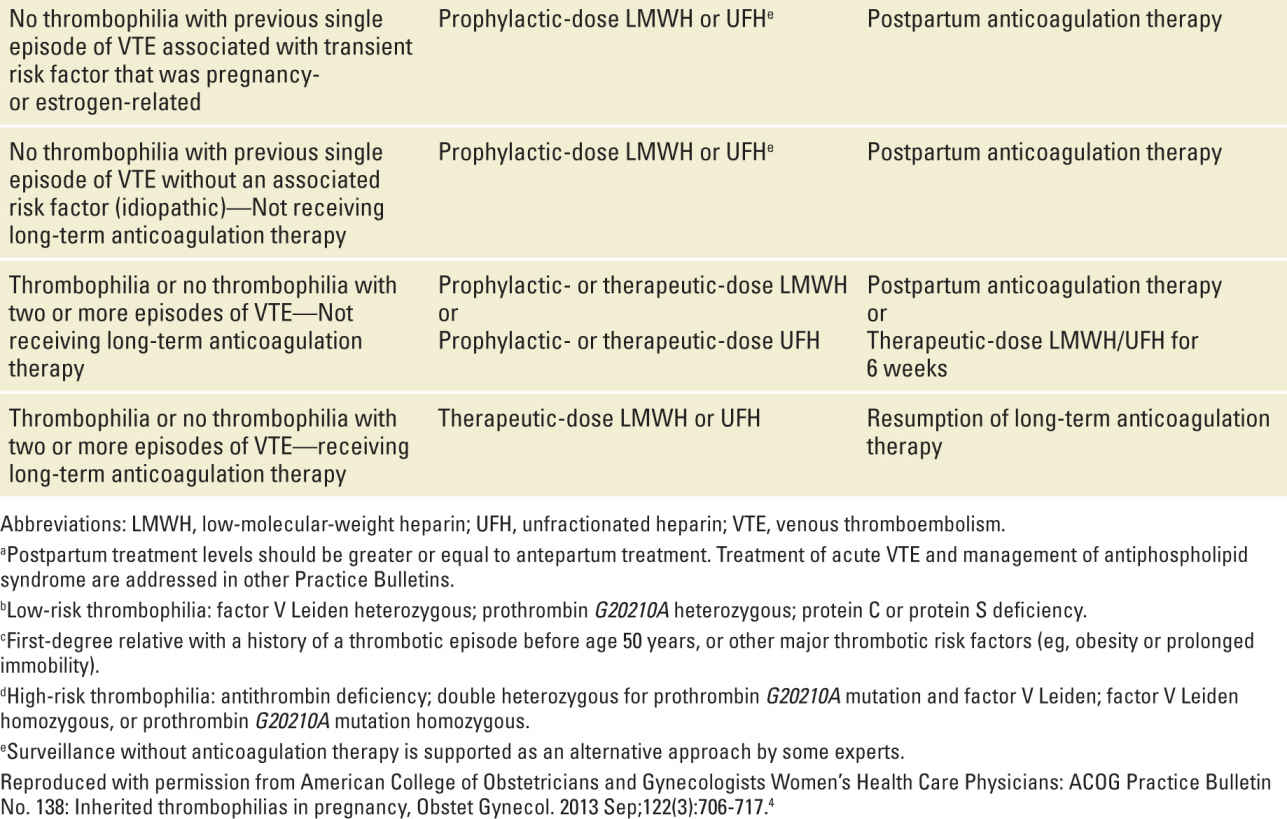
The goal of treatment of women with thrombophilias during pregnancy is to prevent thromboembolism. This requires an assessment of the actual risk for VTE and requires knowledge of the type of thrombophilia, whether or not the individual had a prior VTE, the circumstances of that VTE, and the family history of VTE (and thrombophilia). Typical treatment options include either prophylactic or therapeutic (full anticoagulant) doses of either unfractionated heparin (UH) or low-molecular-weight heparin (LMWH) administered antepartum, intrapartum, and/or postpartum. Dosing should take into account the altered pharmacokinetics of pregnancy. Potential dosing regimens for prophylactic and therapeutic doses of anticoagulant therapy are shown in Table 25-3.
TABLE 25-3 | Anticoagulation Regimen Definitions |
It is noteworthy that the efficacy of anticoagulant therapy during pregnancy has never been proven in appropriately designed trials. Treatment recommendations are based primarily on cohort trials demonstrating the probability of VTE during pregnancy and the puerperium. Anticoagulation is advised in cases with substantially increased risk of VTE. The risk of VTE is considerably higher postpartum than antepartum. Thus, thromboprophylaxis is more strongly advised after delivery than antepartum, especially in women undergoing cesarean delivery. Typically this is done for six weeks postpartum, unless there is an indication for more prolonged anticoagulation. If the patient prefers, warfarin is a safe option for anticoagulant therapy after delivery.
Intrapartum management can be challenging because the risk of thrombosis must be weighed against a desire for neuraxial anesthesia and the risk of hemorrhage associated with vaginal lacerations and cesarean delivery. In most cases, the risk of VTE is low enough so that anticoagulant therapy may be withheld during delivery. It can usually be restarted 6 hours after delivery.
Prophylactic doses of UH can be used in high-risk cases and intravenous UH in very high-risk circumstances such as very recent VTE. AT III concentrate may be appropriate for women with AT III deficiency.
Given the desire to control the risk of hemorrhage and for safe regional anesthesia, it is reasonable to offer women elective induction of labor after 39 weeks gestation. It also is worthwhile to consider switching from LMWH to UH at 36 weeks gestation in women who may spontaneously labor and who strongly desire neuraxial anesthesia. Although UH does not necessarily pose a higher risk of bleeding than LMWH, most anesthesiologists will not place an epidural catheter in women receiving anticoagulant therapy without either waiting 12 to 24 hours depending upon the dose or documenting a normal clotting assay. An activated partial thromboplastin test, which assesses anticoagulation in women taking UH, can be accomplished rapidly in most hospitals. In contrast, an antifactor Xa assay, which assesses anticoagulation in people taking LMWH, cannot be rapidly obtained in most settings. Thus, a patient is less likely to be denied access to regional anesthesia if she is taking UH at the time of delivery. Women should be advised to stop taking UH or LMWH in the setting of labor or rupture of membranes. Protamine sulfate can be used to reverse the anticoagulant effect of UH (and in part LMWH) in cases of acute hemorrhage. Pneumatic compression boots are a reasonable choice for all women with thrombophilias in labor or undergoing cesarean.
Heparins have several adverse effects, although they are relatively safe and pose no fetal risks. The most common is minor irritation at the site of injection. Although it is uncommon in young women, one of the most serious risks is thrombosis associated with heparin-induced thrombocytopenia (HIT). Platelet count should be assessed every 2 to 3 days from 4 to 10 days after initiating prophylactic or therapeutic UH or therapeutic LMWH. Treatment should be stopped in the setting of thrombocytopenia and HIT documented with antiplatelet factor 4 antibodies and serotonin release assay. Prolonged treatment with heparin, especially UH is associated with osteopenia. Although of unproven efficacy, it is reasonable to advise axial skeleton weight-bearing exercise and vitamin D and calcium supplementation in women taking UH.
Table 25-4 outlines current recommendations for anticoagulant therapy during pregnancy and postpartum in women with thrombophilias. These are taken from a recent ACOG practice bulletin.4 In many circumstances there is more than one option (eg, surveillance or thromboprophylaxis). This illustrates the fact that much of this is “expert” opinion and that clear data are lacking for many scenarios. Clinicians should be alert to new data from ongoing studies and updates and modifications to these guidelines as they become apparent.
TABLE 25-4 | Recommended Thromboprophylaxis for Pregnancies Complicated by Inherited Thrombophiliasa |
ANTIPHOSPHOLIPID SYNDROME
Medical and Obstetric Complications
Antiphospholipid antibodies (aPL) have been associated with numerous medical and obstetric complications. The most serious is thromboembolism. In contrast to most thrombophilias, aPL are strongly associated with both venous and arterial thromboembolism. The majority (about two-thirds) of events are venous. The most common site is the lower extremity but up to 50% are pulmonary emboli. In the general population, about 2% of individuals with VTE will have aPL.23 Cerebrovascular accidents are the most frequent type of arterial thrombosis. These include amaurosis fugax and transient ischemic attacks. Both arterial and venous thrombosis may occur in atypical locations. Untreated people with aPL and prior thrombosis have about a 25% yearly recurrence risk. The risk is much less (<1%) in asymptomatic individuals with positive tests for aPL. Pregnancy substantially increases the thrombotic risk in women with aPL. The risk is estimated to be between 5% and 12% in untreated women.24,25
aPL are closely linked to systemic lupus erythematosus (SLE). Many women with SLE will have APS and vice versa. Autoimmune thrombocytopenia also is associated with aPL and occurs in 40% to 50% of cases. It is difficult to distinguish from idiopathic autoimmune thrombocytopenic purpura but fortunately is treated in a similar fashion. Other less common conditions strongly associated with aPL include livedo reticularis, chorea gravidarum, autoimmune hepatitis, pyoderma-like leg ulcers, cardiac valve lesions, and transverse myelitis. Any of these conditions should prompt testing for aPL.
Catastrophic APS refers to a rare but serious condition that is characterized by multiple thromboses of both small and large vessels. Patients suffer severe cardiopulmonary insufficiency, renal failure, and fever. Symptoms are thought to be due to multiple thromboses. The condition may occur postpartum.
The best-known obstetric complication associated with aPL is pregnancy loss. Although aPL are associated with all types of pregnancy loss, the association is strongest for losses later in gestation (fetal death). In one cohort of women with recurrent losses, 50% of losses were fetal deaths in women with aPL compared to only 10% in women without aPL.26 It is important to recognize that there are numerous causes of pregnancy loss other than APS (eg, genetic abnormalities) and that pregnancy loss is common. Thus, it is necessary to exclude other potential causes of pregnancy loss before attributing losses to APS. aPL have been implicated in 5% to 10% of cases of recurrent pregnancy loss. In contrast, they are not associated with sporadic pregnancy loss. Few studies have been conducted in women with late fetal loss. The risk for stillbirth was increased three- to fivefold in women with positive tests for aPL in a recent population-based study.27 However, APS accounts for only a few percent of stillbirths, underscoring the need to evaluate other causes of death.28
Live births in women with aPL often result in obstetric complications due to placental insufficiency, such as preeclampsia, FGR, and abnormal fetal testing. In turn, these conditions increase the risk for medically indicated preterm birth. Even with treatment, up to 50% of women with APS develop preeclampsia and up to a third have FGR.24,25 However, because preeclampsia and FGR are common, APS only accounts for a small percentage of cases of preeclampsia and FGR.
APS is defined by specified clinical criteria with confirmatory laboratory testing. Criteria for APS are shown in Table 25-5. Obstetric criteria include recurrent early pregnancy loss, otherwise unexplained fetal death, and severe placental insufficiency resulting in preterm birth before 34 weeks gestation. These criteria will continue to evolve as more is learned about aPL and associated clinical conditions.
TABLE 25-5 | Revised Classification Criteria for the Antiphospholipid Syndrome |



