The likelihood of developing a thrombosis during pregnancy is especially increased in women with certain genetic risk factors. Indeed—and likely related—after personal history of thrombosis, the next most important individual risk factor is thrombophilia. An estimated 20 to 50 percent of women who develop a venous thrombosis during pregnancy or postpartum have an identifiable underlying genetic disorder (American College of Obstetricians and Gynecologists, 2011).
THROMBOPHILIAS
Several important regulatory proteins act as inhibitors in the coagulation cascade. Normal values for many of these proteins during pregnancy are found in the Appendix (p. 1288). Inherited or acquired deficiencies of these inhibitory proteins are collectively referred to as thrombophilias. These can lead to hypercoagulability and recurrent venous thromboembolism. Although these disorders are collectively present in about 15 percent of white European populations, they are responsible for approximately 50 percent of all thromboembolic events during pregnancy (Lockwood, 2002; Pierangeli, 2011). Some aspects of the more common inherited thrombophilias are summarized in Table 52-2 and Figure 52-1.
TABLE 52-2. Inherited Thrombophilias and Their Association with Venous Thromboembolism (VTE) in Pregnancy
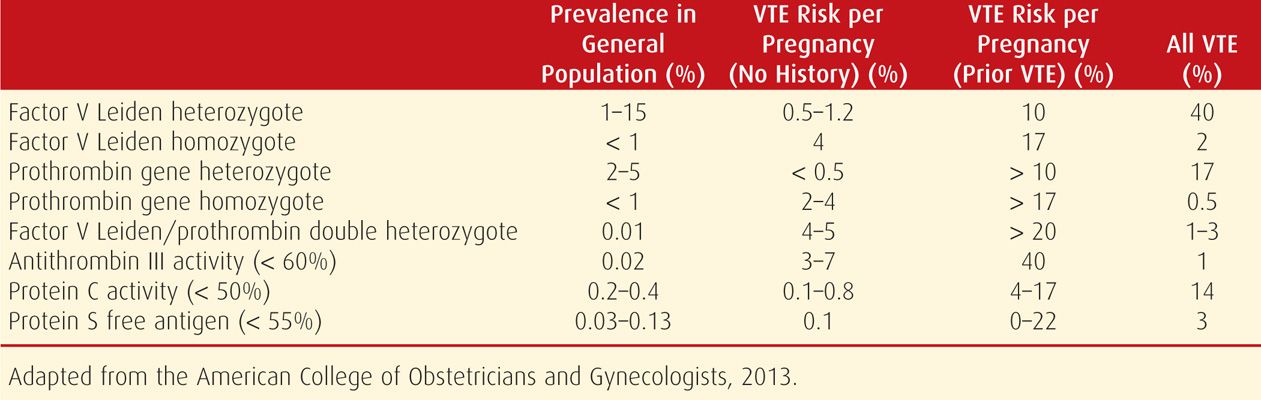
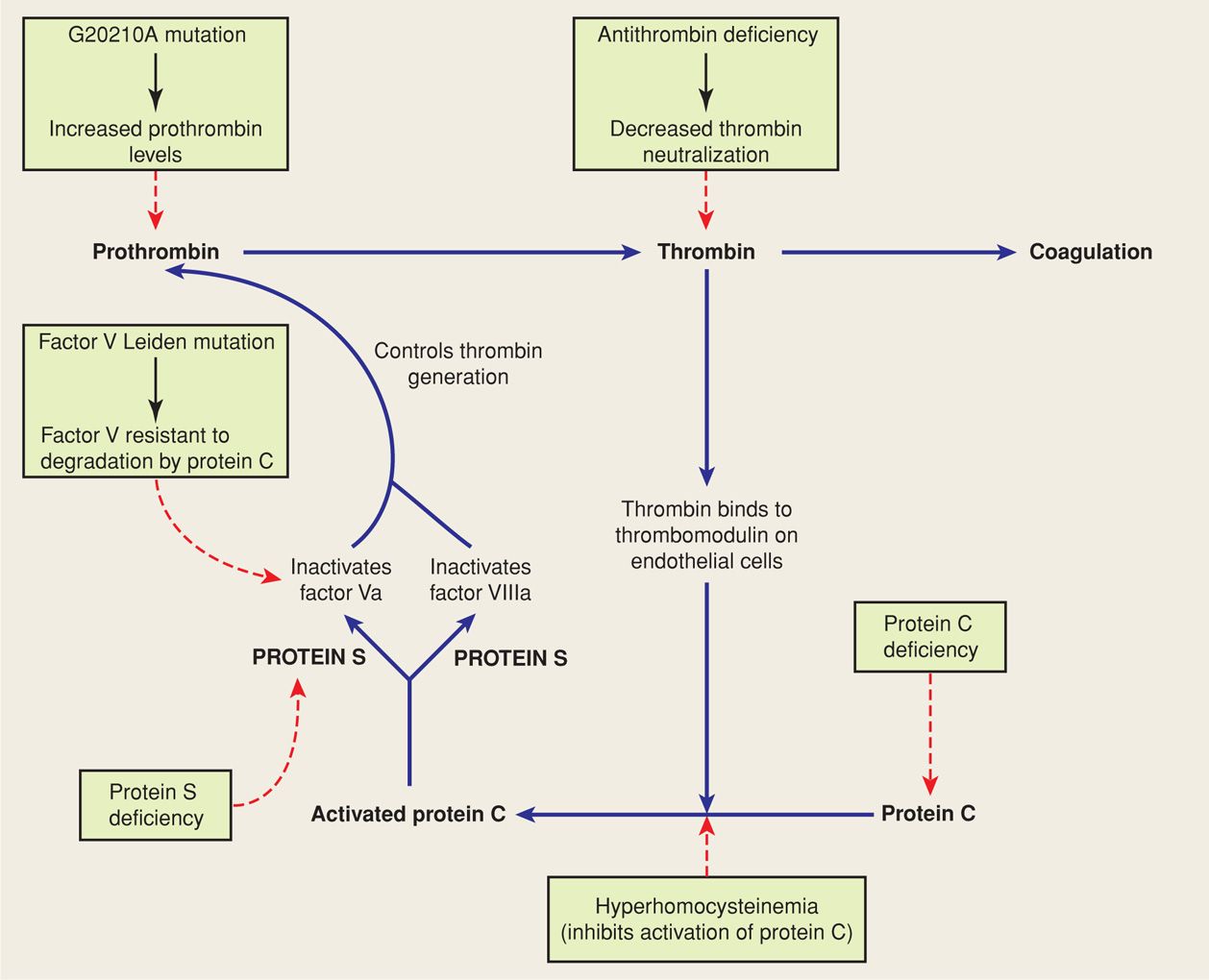
FIGURE 52-1 Overview of the inherited thrombophilias and their effect(s) on the coagulation cascade. (Adapted from Seligsohn, 2001.)
 Inherited Thrombophilias
Inherited Thrombophilias
Patients with inherited thrombophilic disorders often have a family history of thrombosis. Inherited thrombophilias are also found in up to half of all patients who present with venous thromboembolism before the age of 45 years, particularly in those whose event occurred in the absence of well-recognized risk factors, such as surgery or immobilization, or with minimal provocation such as after a long-distance flight or after taking estrogens. Of greatest significance is a family history of sudden death due to pulmonary embolism or a history of multiple family members requiring long-term anticoagulation therapy because of recurrent thrombosis (Anderson, 2011).
Antithrombin Deficiency
Synthesized in the liver, antithrombin is one of the most important inhibitors of thrombin in clot formation. Antithrombin functions as a natural anticoagulant by binding and inactivating thrombin and the activated coagulation factors IXa, Xa, XIa, and XIIa (Franchini, 2006). Of note, the rate of antithrombin interaction with its target proteases is accelerated by heparin (Anderson, 2011). Antithrombin deficiency may result from hundreds of different mutations that are almost always autosomal dominant. Type I deficiency is the result of reduced synthesis of biologically normal antithrombin, and type II deficiency is characterized by normal levels of antithrombin with reduced functional activity (Anderson, 2011). Homozygous antithrombin deficiency is lethal (Katz, 2002).
Antithrombin deficiency is rare—it affects approximately 1 in 2000 to 5000 individuals, and it is the most thrombogenic of the heritable coagulopathies. Indeed, the thrombosis risk during pregnancy among antithrombin-deficient women without a personal or family history is 3 to 7 percent, and it is 11 to 40 percent with such a history (Lockwood, 2012). Specifically, those affected have approximately a 50-percent lifetime risk of venous thromboembolism.
Sabadell and associates (2010) studied the outcomes of 18 pregnancies complicated by antithrombin deficiency. Twelve of these were treated with low-molecular-weight heparin, and six were not treated because antithrombin deficiency had not yet been diagnosed. Three of the untreated patients suffered a thromboembolic episode compared with none in the treated group. Untreated women also had a 50-percent risk of stillbirth and fetal-growth restriction. By comparison, none of the treated women had a stillbirth, and approximately a fourth developed fetal-growth restriction. Seguin and coworkers (1994) reviewed the outcomes of 23 newborns with antithrombin deficiency and described 11 cases of thrombosis and 10 deaths.
Given such risk, affected women are treated during pregnancy with heparin regardless of whether they have had a prior thrombosis. When anticoagulation is necessarily withheld, such as during surgery or delivery, Paidas and colleagues (2013) found that treatment with recombinant human antithrombin protected against venous thromboembolism development in 21 patients with hereditary antithrombin deficiency. Sharpe and associates (2011) described successful use of antithrombin concentrate infusions plus therapeutic anticoagulation in a pregnant woman with antithrombin deficiency who developed a thrombosis during the third trimester despite therapeutic doses of low-molecular-weight heparin.
Protein C Deficiency
When thrombin is bound to thrombomodulin on endothelial cells of small vessels, its procoagulant activities are neutralized. This binding also activates protein C, a natural anticoagulant that in the presence of protein S controls thrombin generation, in part, by inactivating factors Va and VIIIa (see Fig. 52-1). Activated protein C also inhibits the synthesis of plasminogen-activator inhibitor 1 (p. 1032).
Protein C activity is largely unchanged in pregnancy (Appendix, p. 1288). Based on their study of 440 healthy women, however, Said and associates (2010b) found that protein C activity increases modestly but significantly throughout the first half of pregnancy. These investigators speculated that this increase may play a role in maintaining early pregnancy through both anticoagulant and inflammatory regulatory pathways.
More than 100 different autosomal dominant mutations for the protein C gene have been described. The prevalence of protein C deficiency is 2 to 3 per 1000, but many of these individuals do not have a thrombosis history because the phenotypic expression is highly variable (Anderson, 2011). These prevalence estimates correspond with functional activity threshold values of 50 to 60 percent, which are used by most laboratories and which are associated with a six- to 12-fold increased risk for venous thromboembolism (Lockwood, 2012).
Protein S Deficiency
This circulating anticoagulant is activated by protein C, which enhances its capacity to inactiviate factors Va and VIIIa (see Fig. 52-1). Protein S deficiency may be caused by more than 100 different mutations, with an aggregate prevalence of approximately 2 per 1000 (Lockwood, 2012). Protein S deficiency may be measured by antigenically determined free, functional, and total S levels. All three decline substantively during normal gestation, thus the diagnosis in pregnant women—as well as in those taking certain oral contraceptives—is difficult (Archer, 1999). If screening during pregnancy is necessary, threshold values for free protein S antigen levels in the second and third trimesters have been identified at less than 30 percent and less than 24 percent, respectively. Among those with a positive family history, the venous thromboembolism risk in pregnancy has been reported to be 6 to 7 percent (American College of Obstetricians and Gynecologists, 2013).
Conard and coworkers (1990) described thrombosis in 5 of 29 pregnant women with protein S deficiency. One woman had a cerebral vein thrombosis. Similarly, Burneo and colleagues (2002) reported maternal cerebral vein thrombosis at 14 weeks’ gestation. Neonatal homozygous protein C or S deficiency is usually associated with a severe clinical phenotype known as purpura fulminans. This is characterized by extensive thromboses in the microcirculation soon after birth leading to skin necrosis (Salonvaara, 2004).
Activated Protein C Resistance—Factor V Leiden Mutation
The most prevalent of the known thrombophilia syndromes, this condition is characterized by resistance of plasma to the anticoagulant effects of activated protein C. A number of mutations have been described, but the most common is the factor V Leiden mutation, which was named after the city in which it was described. This missense mutation in the factor V gene results from a substitution of glutamine for arginine at position 506 in the factor V polypeptide, which gains resistance to degradation by activated protein C. The unimpeded abnormal factor V protein retains its procoagulant activity and predisposes to thrombosis (see Fig. 52-1).
Heterozygous inheritance for factor V Leiden is the most common heritable thrombophilia. It is found in 3 to 15 percent of select European populations and 3 percent of African Americans, but it is virtually absent in African blacks and Asians (Lockwood, 2012). As shown in Table 52-2, women who are heterozygous for factor V Leiden account for approximately 40 percent of venous thromboembolism cases during pregnancy. However, the actual risk among pregnant women who are heterozygous and who do not have a personal history or a first-degree relative with a thrombotic episode before age 50 years is 5 to 12 per 1000. In contrast, this risk increases to at least 10 percent among women with a personal or family history. Pregnant women who are homozygous without a personal or family history have a 1- to 4-percent risk for venous thromboembolism, whereas those with such a history have an approximately 17-percent risk (American College of Obstetricians and Gynecologists, 2013).
As described later (p. 1034), diagnosis during pregnancy is performed by DNA analysis for the mutant factor V gene. This is because bioassay is confounded by the fact that resistance is normally increased after early pregnancy because of alterations in other coagulation proteins (Walker, 1997). Of note, activated protein C resistance can also be caused by antiphospholipid syndrome, which is described later (p. 1033) and also detailed in Chapter 59 (p. 1173) (Eldor, 2001; Saenz, 2011).
To assess the prognostic significance of maternal factor V Leiden mutation during pregnancy, Kjellberg and colleagues (2010) compared the outcomes of 491 carriers with 1055 controls. All three of the thromboembolic events occurred among the carriers. But, there were no differences in preterm birth, birthweight, or hypertensive complications between the two groups. Similarly, Hammerová and coworkers (2011) found that adverse pregnancy events were not increased among women with heterozygous mutations. In a meticulously executed prospective observational study of approximately 5000 women conducted by the Maternal-Fetal Medicine Units Network, Dizon-Townson and associates (2005) found that the heterozygous mutant gene incidence was 2.7 percent. Of three pulmonary emboli and one deep-vein thrombosis cases—a rate of 0.8 per 1000 pregnancies—none were among these carriers. There was no increased risk of preeclampsia, placental abruption, fetal-growth restriction, or pregnancy loss in heterozygous women. The investigators concluded that universal prenatal screening for the Leiden mutation and prophylaxis for carriers without a prior venous thromboembolism is not indicated. Finally, Clark and colleagues (2002) concluded that such routine prenatal screening was not cost effective.
Prothrombin G20210A Mutation
This missense mutation in the prothrombin gene leads to excessive accumulation of prothrombin, which then may be converted to thrombin. As with factor V Leiden, a personal history or a family history of venous thromboembolism in a first-degree relative before age 50 years increases the risk of venous thromboembolism during pregnancy (see Table 52-2). For a heterozygous carrier with such a history, the risk exceeds 10 percent. Without such a history, heterozygous carriers of the mutation have less than a 1-percent risk of venous thromboembolism during pregnancy (American College of Obstetricians and Gynecologists, 2013).
Homozygous patients or those who coinherit a G20210A mutation with a factor V Leiden mutation have an even greater thromboembolism risk. Stefano and associates (1999) performed a retrospective cohort study of 624 nonpregnant patients with one prior episode of deep-vein thrombosis. They found that those doubly heterozygous individuals had a 2.6-fold increased risk of recurrence relative to those with the heterozygous Leiden mutation alone. They concluded that carriers with both mutations are candidates for lifelong anticoagulation after a first thrombotic episode.
In a secondary analysis of the Maternal-Fetal Medicine Units Network study described earlier, Silver and coworkers (2010) tested nearly 4200 women for the prothrombin G20210A mutation. A total of 157—or 3.8 percent—of the women carried the mutation, and only one of these was homozygous. Carriers had similar rates of pregnancy loss, preeclampsia, growth restriction, and placental abruption compared with noncarriers. The three thromboembolic events occurred in women who tested negative for the mutation.
Hyperhomocysteinemia
The most common cause of elevated homocysteine is the C667T thermolabile mutation of the enzyme 5, 10-methylene-tetrahydrofolate reductase (MTHFR). Inheritance is autosomal recessive. Elevated homocysteine levels may also result from deficiency of one of several enzymes involved in methionine metabolism and from correctible nutritional deficiencies of folic acid, vitamin B6, or vitamin B12 (Hague, 2003; McDonald, 2001). During normal pregnancy, mean homocysteine plasma concentrations are decreased (López-Quesada, 2003; McDonald, 2001). Thus, to make a diagnosis during pregnancy, Lockwood (2002) recommends a fasting threshold of > 12 μmol/L to define hyperhomocysteinemia.
Although hyperhomocysteinemia was previously reported to be a modest risk factor for venous thromboembolism, more recent data indicate that an elevated homocysteine level is actually a weak risk factor (American College of Obstetricians and Gynecologists, 2013). In an interesting metaanalysis, den Heijer and colleagues (2005) found that international studies of MTHFR polymorphisms were collectively associated with slightly increased significant risks for thrombosis—odds ratios 1.15 to 1.6. In contrast, studies conducted in North America collectively demonstrated no such association. The authors speculated that folic acid supplementation could explain the difference. Recall that folic acid serves as a cofactor in the remethylation reaction of homocysteine to methionine. Similarly, the American College of Chest Physicians concluded that the lack of an association with thromboembolism could reflect the physiological reductions in homocysteine levels associated with pregnancy and the effects of widespread prenatal folic acid supplementation (Bates, 2012).
In a follow-up study of 167 women who developed a venous thromboembolism during pregnancy and 128 controls, Kovac and associates (2010) found no difference in the prevalence of MTHFR C677T homozygosity between the two groups. The American College of Obstetricians and Gynecologists (2013) has concluded that there is insufficient evidence to support assessment of MTHFR polymorphisms or measurement of fasting homocysteine levels in the evaluation for venous thromboembolism.
Other Thrombophilia Mutations
A number of potentially thrombophilic polymorphisms are being discovered at an ever-increasing rate. Unfortunately, information regarding the prognostic significance of such newly discovered mutations is limited. For example, protein Z is a vitamin K-dependent protein that serves as a cofactor in factor Xa inactivation. Studies in nonpregnant patients have found that low protein Z levels are associated with an increased thromboembolism risk (Santacroce, 2006). Similarly, plasminogen activator inhibitor type 1 (PAI-1) is an important regulator of fibrinolysis. Certain polymorphisms in the gene promoter have been associated with small increased venous thromboembolism risks. These thrombophilias and others, including alternative mutations in the factor V gene and activity-enhancing mutations in various clotting factor genes, appear to exert little independent risk for venous thromboembolism. And although they may exacerbate risk among patients when coinherited with other thrombophilias, the American College of Obstetricians and Gynecologists (2013) has concluded that there is insufficient evidence to recommend screening.
As an interesting aside, Galanaud and coworkers (2010) hypothesized that a paternal thrombophilia could increase the risk of a maternal thromboembolism. Specifically, these investigators found that a paternal thrombophilia—the PROCR 6936G allele—affects the endothelial protein C receptor. This receptor is expressed by villous trophoblast and thus is exposed to maternal blood. Although this research is preliminary, it could help explain the pathogenesis of recurrent idiopathic thromboses in pregnant women.
 Acquired Thrombophilias
Acquired Thrombophilias
Some examples of acquired hypercoagulable states include antiphospholipid syndrome (Chap. 59, p. 1173), heparin-induced thrombocytopenia (p. 1040), and cancer (Chap. 63, p. 1219).
Antiphospholipid Antibodies
These autoantibodies are detected in approximately 2 percent of patients who have nontraumatic venous thrombosis. The antibodies are directed against cardiolipin(s) or against phospholipid-binding proteins such as β2-glycoprotein I. They are commonly—but not always—found in patients with systemic lupus erythematosus and are described in detail in Chapter 59 (p. 1169). Women with moderate-to-high levels of these antibodies may have antiphospholipid syndrome, which, as summarized by the American College of Obstetricians and Gynecologists (2012), is defined by a number of clinical features. In addition to vascular thromboses, these include: (1) at least one otherwise unexplained fetal death at or beyond 10 weeks; (2) at least one preterm birth before 34 weeks because of eclampsia, severe preeclampsia, or placental insufficiency; or (3) at least three unexplained consecutive spontaneous abortions before 10 weeks.
In these women, thromboembolism—either venous or arterial—most commonly involves the lower extremities. Importantly, the syndrome should also be considered in women with thromboses in unusual sites, such as the portal, mesenteric, splenic, subclavian, axillary, and cerebral veins. Antiphospholipid antibodies predispose to arterial thromboses, which may also occur in relatively unusual locations, such as the retinal, subclavian, brachial, or digital arteries. The thrombotic mechanisms associated with antiphospholipid syndrome have recently been reviewed by Giannakopoulos and Krilis (2013).
The thrombosis risk increases significantly during pregnancy in women with antiphospholipid syndrome. Indeed, up to 25 percent of thrombotic events in women with antiphospholipid syndrome occur during pregnancy or in the puerperium. Looking at this a different way, women with antiphospholipid syndrome have a 5- to 12-percent risk of thrombosis during pregnancy or the puerperium (American College of Obstetricians and Gynecologists, 2012).
 Thrombophilias and Pregnancy Complications
Thrombophilias and Pregnancy Complications
Attention has been directed toward possible relationships between inherited thrombophilias and pregnancy complications other than thromboses. Summarized in Table 52-3 are the findings of 25 studies systematically reviewed by Robertson and associates (2005). These were incorporated into the most recent recommendations of the American College of Chest Physicians (Bates, 2012). Importantly, the considerable heterogeneity and wide confidence intervals illustrate the uncertainty of these associations.
TABLE 52-3. Obstetrical Complications Associated with Thrombophilias
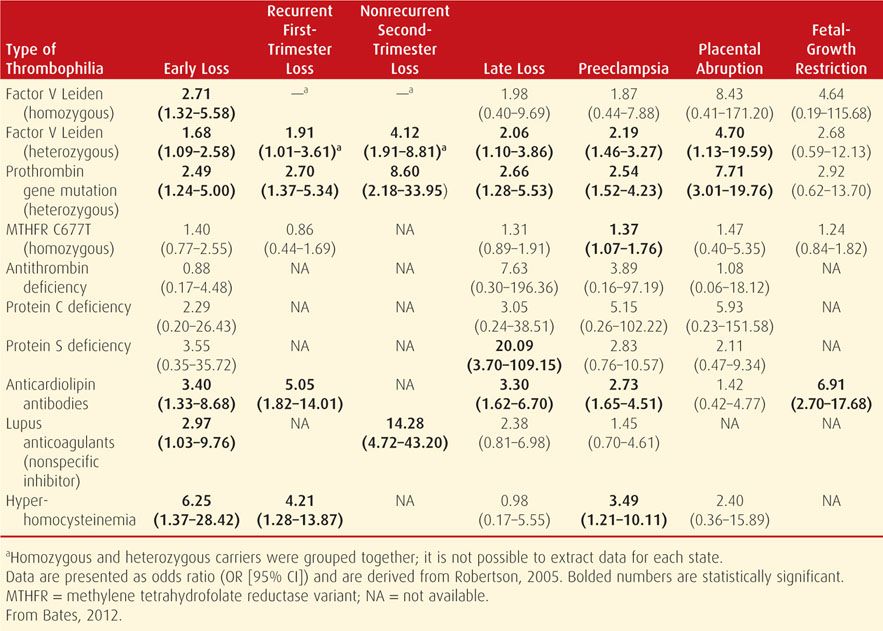
More recent investigations continue to underscore the heterogeneity of results. For example, Kahn and coworkers (2009) found no increased risk for early-onset or severe preeclampsia in women with factor V Leiden mutation, prothrombin G20210A mutation, MTHFR C677T polymorphism, or hyperhomocysteinemia. Said and associates (2010a) prospectively screened more than 2000 healthy nulliparous women for factor V Leiden, prothrombin gene mutation, MTHFR C677T, MTHFR A1298C, and thrombomodulin polymorphism. Women who carried the prothrombin gene mutation had a 3.6-fold increased risk of adverse pregnancy outcome, including severe preeclampsia, fetal-growth restriction, placental abruption, or stillbirth. But, none of the other polymorphisms conferred an increased risk of these adverse outcomes. Moreover, this group of investigators found no association between the PAI-1 4G/5G polymorphism and adverse pregnancy outcome (Said, 2012). Similarly, based on their prospective study of 750 pregnancies complicated by stillbirth, Korteweg and colleagues (2010) concluded that routine thrombophilia testing after fetal death is inadvisable.
Because of uncertainties associated with the magnitude of risk as well as any benefits of prophylaxis given to prevent pregnancy complications in women with heritable thrombophilias, it remains unproven that screening is in the best interest of these women. The American College of Obstetricians and Gynecologists (2013) has concluded that a definitive causal link cannot be made between inherited thrombophilias and adverse pregnancy outcomes. Similarly, the American College of Chest Physicians recently concluded that it was unclear whether screening for inherited thrombophilias is prudent in women with pregnancy complications (Bates, 2012). In contrast, and as shown in Table 52-3 and detailed in Chapter 59 (p. 1174), the association between antiphospholipid syndrome and adverse pregnancy outcomes—including fetal loss, recurrent pregnancy loss, and preeclampsia—is much stronger.
 Thrombophilia Screening
Thrombophilia Screening
Given the high incidence of thrombophilia in the population and the low incidence of venous thromboembolism, universal screening during pregnancy is not cost effective (Carbone, 2010). Thus, a selective screening strategy is required. The American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) recommend that thrombophilia screening be considered in the following clinical circumstances: (1) a personal history of venous thromboembolism that was associated with a nonrecurrent risk factor such as fractures, surgery, and/or prolonged immobilization; and (2) a first-degree relative (parent or sibling) with a history of high-risk thrombophilia or venous thromboembolism before age 50 years in the absence of other risk factors.
As described earlier, the American College of Obstetricians and Gynecologists (2013) has concluded that testing for inherited thrombophilias in women who have experienced recurrent fetal loss or placental abruption is not recommended because there is insufficient clinical evidence that antepartum heparin prophylaxis prevents recurrence. Similarly, testing is not recommended for women with a history of fetal-growth restriction or preeclampsia. The American College of Chest Physicians also recommends against screening women with prior pregnancy complications (Bates, 2012). As discussed in Chapter 59, however, screening for antiphospholipid antibodies may be appropriate in women who have experienced a fetal loss.
Screening Tests
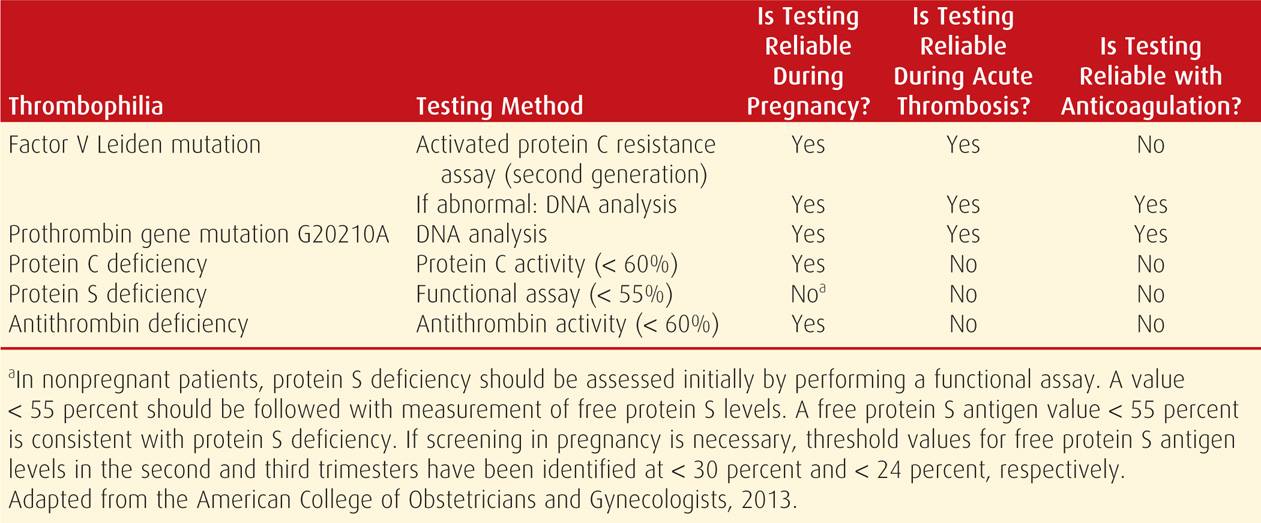
Methods of screening for the more common inherited thrombophilias are shown in Table 52-4. Whenever possible, laboratory testing should be performed at least 6 weeks after the thrombotic event, while the patient is not pregnant, and when she is not receiving anticoagulation or hormonal therapy. Because of the lack of association between methylenetetrahydrofolate reductase (MTHFR) gene mutations—the most common cause of hyperhomocysteinemia—and adverse pregnancy outcomes, screening with fasting homocysteine levels or MTHFR mutation analyses is not recommended (American College of Obstetricians and Gynecologists, 2013).
TABLE 52-4. Inherited Thrombophilia Testing
DEEP-VEIN THROMBOSIS
 Clinical Presentation
Clinical Presentation
During pregnancy, most venous thromboses are confined to the deep veins of the lower extremity. Approximately 70 percent of cases are located in the iliofemoral veins without involvement of the calf veins. Isolated iliac vein and calf vein thromboses occur in approximately 17 and 6 percent of cases, respectively (Chan, 2010).
The signs and symptoms vary greatly and depend on the degree of occlusion and the intensity of the inflammatory response. Most cases during pregnancy are left sided. Ginsberg and coworkers (1992) reported that 58 of 60 antepartum women—97 percent—had left leg thromboses. Blanco-Molina and coworkers (2007) reported left-leg involvement in 78 percent. Our experiences at Parkland Hospital are similar—approximately 90 percent of lower extremity thromboses involved the left leg. Greer (2003) hypothesizes that this results from compression of the left iliac vein by the right iliac and ovarian artery, both of which cross the vein only on the left side. Yet, as described in Chapter 53 (p. 1051), the ureter is compressed more on the right side!
Classic thrombosis involving the lower extremity is abrupt in onset, and there is pain and edema of the leg and thigh. The thrombus typically involves much of the deep-venous system to the iliofemoral region. Occasionally, reflex arterial spasm causes a pale, cool extremity with diminished pulsations. Conversely, there may be appreciable clot, yet little pain, heat, or swelling. Importantly, calf pain, either spontaneous or in response to squeezing or to Achilles tendon stretching—Homans sign—may be caused by a strained muscle or contusion. Between 30 and 60 percent of women with a confirmed lower-extremity acute deep-vein thrombosis have an asymptomatic pulmonary embolism (p. 1041).
 Diagnosis
Diagnosis
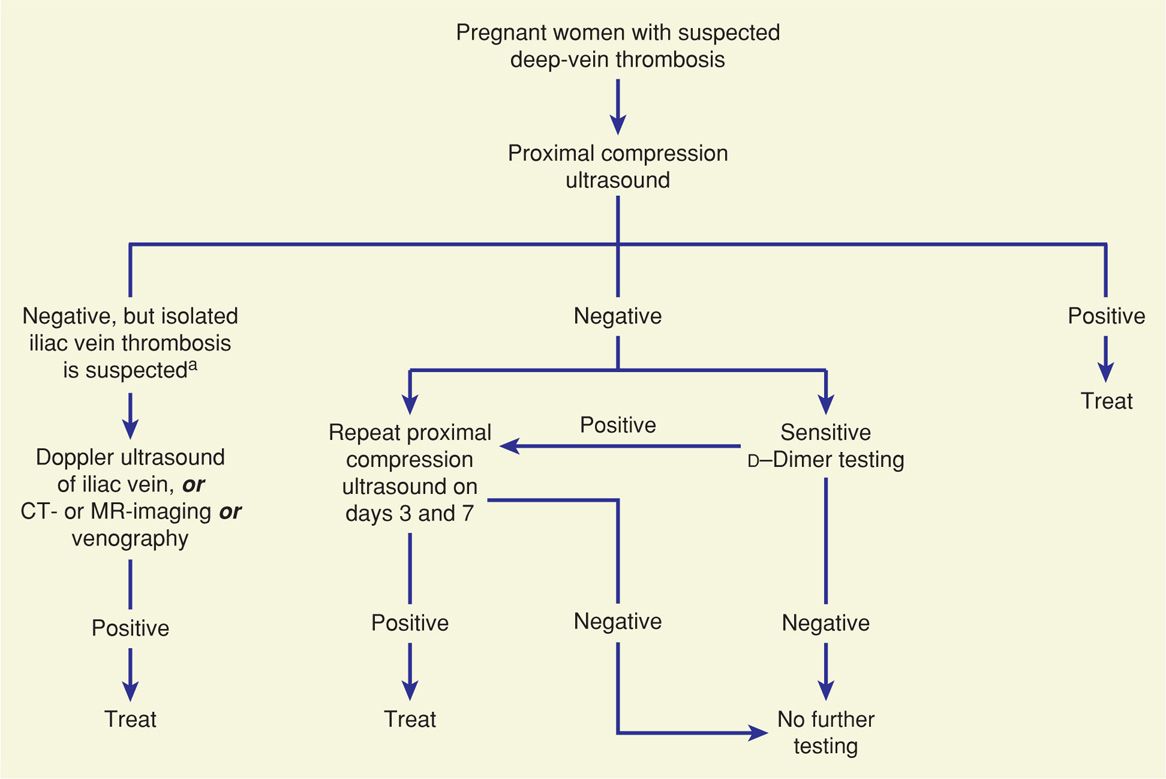
Clinical diagnosis of deep-vein thrombosis is difficult, and thus other methods are imperative for confirmation. In one study of pregnant women, the clinical diagnosis was confirmed in only 10 percent (Hull, 1990). Shown in Figure 52-2 is one diagnostic algorithm recommended by the American College of Chest Physicians that can be used for evaluation of pregnant women (Guyatt, 2012). With a few modifications, we follow a similar evaluation at Parkland Hospital.
FIGURE 52-2 Algorithm for evaluation of suspected deep-vein thrombosis in pregnancy. CT = computed tomography; MR = magnetic resonance. (Adapted from the American College of Chest Physicians, Guyatt, 2012.) aSigns and symptoms include swelling of the entire leg, with or without flank, buttock, or back pain.
Compression Ultrasonography
In pregnant women with suspected deep-vein thrombosis, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) recommend compression ultrasonography of the proximal veins as the initial diagnostic test. According to the American College of Chest Physicians, this noninvasive technique is currently the most-used first-line test to detect deep-vein thrombosis (Guyatt, 2012). The diagnosis is based on the noncompressibility and typical echoarchitecture of a thrombosed vein.
For nonpregnant patients with suspected thrombosis, the safety of withholding anticoagulation has been established for those who have normal serial compression examinations over a week (Birdwell, 1998; Heijboer, 1993). Specifically, isolated calf thromboses that extend into the proximal veins in about a fourth of patients will do so within 1 to 2 weeks of presentation. Moreover, these are usually detected by serial ultrasonographic compression.
In pregnant women, the important caveat is that normal findings with venous ultrasonography results do not always exclude a pulmonary embolism. This is because the thrombosis may have already embolized or because it arose from iliac or other deep-pelvic veins, which are less accessible to ultrasound evaluation (Goldhaber, 2004). Thrombosis associated with pulmonary embolism during pregnancy frequently originates in the iliac veins. Although serial investigations are recommended by many, Le Gal and colleagues (2012) recently studied the use of nonserial proximal and distal compression ultrasonography in 226 pregnant and postpartum women with suspected deep-vein thrombosis. Deep-vein thrombosis was diagnosed in 10 percent. Of the 177 women without a deep-vein thrombosis and who were not anticoagulated, two had an objectively confirmed thrombosis diagnosed within three months. Thus, these preliminary data suggest that a negative single complete compression ultrasonography study may safely exclude the diagnosis of deep-vein thrombosis in most pregnant women.
Magnetic Resonance Imaging
This imaging technique allows excellent delineation of anatomical detail above the inguinal ligament. Thus, in many cases, magnetic resonance (MR) imaging is immensely useful for diagnosis of iliofemoral and pelvic vein thrombosis. The venous system can also be reconstructed using MR venography as discussed in Chapter 46 (Fig. 46-5, p. 936). Erdman and associates (1990) reported that MR imaging was 100-percent sensitive and 90-percent specific for detection of venographically proven deep-vein thrombosis in nonpregnant patients. Importantly, almost half of those without deep-vein thrombosis were found to have nonthrombotic conditions that included cellulitis, myositis, edema, hematomas, and superficial phlebitis.
Khalil and coworkers (2012) used magnetic resonance venography to study the natural history of pelvic vein thrombosis after vaginal delivery. Among the 30 asymptomatic patients who were all within four days of delivery, 30 percent had a definitive thrombosis in either the iliac or ovarian veins, and another 37 percent had a suspected thrombosis. Our experience with hundreds of postpartum MR scans does not support these findings. Thus, although the clinical significance of their findings is uncertain, it seems clear that some degree of pelvic vein intraluminal filling defect may be a normal finding.
D-Dimer Screening Tests
These specific fibrin degradation products are generated when fibrinolysin degrades fibrin, as occurs in thromboembolism (Chap. 41, p. 809). Their measurement is frequently incorporated into diagnostic algorithms for venous thromboembolism in nonpregnant patients (Kelly, 2002; Wells, 2003). Screening with the D-dimer test in pregnancy, however, is problematic for a number of reasons. As shown in the Appendix (p. 1288), depending on assay sensitivity, D-dimer serum levels increase with gestational age along with substantively elevated plasma fibrinogen concentrations (McCrae, 2014). Levels are also affected by multifetal gestation and cesarean delivery (Morikawa, 2011). In a serial study of 50 healthy women, Kline and colleagues (2005) found that D-dimer levels increased progressively during pregnancy. Also, 22 percent of women in midpregnancy and no women in the third trimester had a D-dimer concentration below 0.50 mg/L—a conventional threshold used to exclude thromboembolism. D-Dimer concentrations can also be elevated in certain pregnancy complications such as placental abruption, preeclampsia, and sepsis syndrome. For these reasons, their use during pregnancy remains uncertain, but a negative D-dimer test should be considered reassuring (Lockwood, 2012; Marik, 2008).
Venography
Invasive contrast venography is the gold standard to exclude lower extremity deep-vein thrombosis (Chunilal, 2001). It has a negative-predictive value of 98 percent, and as discussed in Chapter 46 (p. 932), fetal radiation exposure without shielding is approximately 3 mGy (Nijkeuter, 2006). That said, venography is associated with significant complications, including thrombosis, and it is time consuming and cumbersome. Because of this, noninvasive methods are used primarily to confirm the diagnosis, and venography is seldom used today.
 Management
Management
Optimal management of venous thromboembolism during pregnancy has not undergone major clinical study to provide evidence-based practices. There is, however, consensus for treatment with anticoagulation and limited activity. If thrombophilia testing is performed, it is done before anticoagulation because heparin induces a decline in antithrombin levels, and warfarin decreases protein C and S concentrations (Lockwood, 2002).
Anticoagulation is initiated with either unfractionated or low-molecular-weight heparin. Although either type is acceptable, most recommend one of the low-molecular-weight heparins. In its recently revised guidelines, for example, the American College of Chest Physicians suggests preferential use of low-molecular-weight heparin during pregnancy because of better bioavailability, longer plasma half-life, more predictable dose response, reduced risks of osteoporosis and thrombocytopenia, and less frequent dosing (Bates, 2012).
During pregnancy, heparin therapy is continued, and for postpartum women, anticoagulation is begun simultaneously with warfarin. Recall that pulmonary embolism develops in as many as 60 percent of patients with untreated venous thrombosis, and anticoagulation decreases this risk to less than 5 percent. In nonpregnant patients, the mortality rate is approximately 1 percent (Douketis, 1998; Pollack, 2011).
Over several days, leg pain dissipates. After symptoms have abated, graded ambulation should be started. Elastic stockings are fitted, and anticoagulation is continued. Recovery to this stage usually takes 7 to 10 days. Graduated compression stockings should be continued for 2 years after the diagnosis to reduce the incidence of postthrombotic syndrome (Brandjes, 1997). This syndrome can include chronic leg paresthesias or pain, intractable edema, skin changes, and leg ulcers.
 Unfractionated Heparin
Unfractionated Heparin
This agent should be considered for the initial treatment of thromboembolism and in situations in which delivery, surgery, or thrombolysis may be necessary (p. 1039) (American College of Obstetricians and Gynecologists, 2011). Unfractionated heparin (UFH) can be administered by one of two alternatives: (1) initial intravenous therapy followed by adjusted-dose subcutaneous UFH given every 12 hours; or (2) twice-daily, adjusted dose subcutaneous UFH with doses adjusted to prolong the activated partial thromboplastin time (aPTT) into the therapeutic range 6 hours postinjection (Bates, 2012). As shown in Table 52-5, the therapeutic dose for subcutaneous UFH is usually 10,000 units or more every 12 hours.
TABLE 52-5. Anticoagulation Regimens
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree