Thermal Regulation
Michael Friedman
Stephen Baumgart
A HISTORICAL PERSPECTIVE
Tarnier was an obstetrician in Paris who first applied modern concepts of incubation to human infants starting around 1830 (1,2). Tarnier’s incubator, the couveuse, has been widely recognized as the first one designed specifically to care for premature babies. Tarnier and his student, Budin, studied premature human incubation into the next century, reporting almost doubled survival in infants born at less than 2 kg. In the United States, commercialization of Tarnier’s and Budin’s designs occurred, and the Rotch Incubator appeared at the Colombian Exposition in Chicago in 1893 (3,4,5). Thereafter, in 1933 Blackfan and Yaglou (6) provided humidity along with air warming within incubators, which improved the stability of infant temperature control. In the 1940s Chappel in Philadelphia added air isolation techniques to incubator care to prevent neonatal septic infections recognized to occur more frequently in humid environments (7,8). In 1958, Silverman and associates (9) challenged the need for humidity in incubators and used higher air temperatures than previously reported to care for an ever smaller premature population surviving with modern techniques.
Toward Defining the Optimal Thermal-Neutral Environment
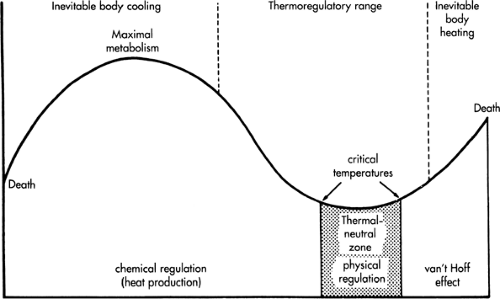
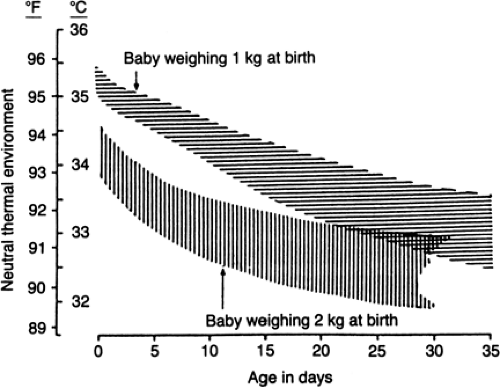
In 1959 Cross and Hill from the United Kingdom described a metabolically neutral temperature for achieving the optimal environmental care of newborn animals and humans, suggesting that poor temperature maintenance resulted in increased metabolic rates of oxygen and substrate consumption (10,11,12) (Fig. 24-1). Physiologic responses to environmental temperature during incubation of premature newborn infants were investigated systematically in 1962 by Brück and coworkers (13,14) from Germany. In 1969 Hey and associates (15,16,17) from the United Kingdom defined the best operational incubator temperatures for preterm babies by describing an algorithm of air and wall temperatures, taking humidification and swaddling (insulation) into account as well. The Hey and Katz nomograms for regulating incubators following preterm birth, growth, and maturation remain the benchmark standard for modern human incubation to date (Fig. 24-2).
THERMAL BALANCE AT THE BEGINNING OF LIFE
Fetal Thermal Regulation
The fetus generates heat during metabolism with cellular proliferation and differentiation, maintenance of intra- and extracellular ion gradients, and transport of nutrients and wastes across cell membranes. Cardiac and skeletal muscle work also generates heat in utero (18). Fetal ovine and human studies suggest that the rate of fetal heat production is about 33 to 47 cal/kg/minute (18,19). Fetal-maternal temperature gradients in mammals and humans have demonstrated that a difference in temperature of only 0.45°C to 0.50°C between the umbilic arterial and venous blood is sufficient to eliminate the majority of metabolic heat via the placental circulation (i.e., by forced convective transfer into the mother’s uterine circulation) (20,21,22). Probably less than 10% to 20% of heat is dissipated from the fetal skin into the amniotic fluid (natural convection and conduction from the uterine wall). The mother additionally serves as a heat reservoir for the fetus, favoring the dissipation of heat as a byproduct of fetal metabolism. Fatal hyperthermia may occur with an elevation of maternal temperature or if the mother is unable to dissipate the excess heat produced during pregnancy. Therefore, pregnant women are advised to avoid prolonged hot baths and exertion on hot and humid days. Maternal fever should be treated aggressively with environmental cooling and with antipyretics and antibiotics when indicated.
Transition in the Delivery Suite
At birth, a newly born infant is immediately exposed to a wet and cold environment. Without intervention, rapid cooling by convection from the neonate’s skin into the cold delivery room air (at least a 10.0°C drop) and by
evaporation at a tremendous rate (0.58 kcal/mL of water loss) may result in a drop of the infant’s body temperature at a rate of 0.2°C to 1.0°C/minute. Although fetal response to cold stress is relatively insensitive prenatally (22,23), increased infant activity (crying with agitated movement characteristic of cold exposure upon birth), vasoconstriction, and nonshivering thermogenesis (shivering is not active in the human newborn) occurs the instant the baby hits the cold air, mediated by the sympathetic nervous system (24). Triggered by temperature sensation of the skin, infant metabolic rate may increase by two- to threefold and thus maintain body temperature for a period of several hours in the term subject before thermogenic reserves of glycogen and brown fat become depleted.
evaporation at a tremendous rate (0.58 kcal/mL of water loss) may result in a drop of the infant’s body temperature at a rate of 0.2°C to 1.0°C/minute. Although fetal response to cold stress is relatively insensitive prenatally (22,23), increased infant activity (crying with agitated movement characteristic of cold exposure upon birth), vasoconstriction, and nonshivering thermogenesis (shivering is not active in the human newborn) occurs the instant the baby hits the cold air, mediated by the sympathetic nervous system (24). Triggered by temperature sensation of the skin, infant metabolic rate may increase by two- to threefold and thus maintain body temperature for a period of several hours in the term subject before thermogenic reserves of glycogen and brown fat become depleted.
Physiology of Neonatal Thermal Response
Brown fat is especially thermogenic in the term newborn, with large reserves located between the scapula, in the axillae and perithymic region, and in the paraspinal and peri-nephric areas. Penetrated extensively by blood vessels, which give it the brown appearance and which conduct heat generated into the central circulation, these adipo-cytes are laden with excess triglyceride stores and numerous mitochondria. With cold stress, the sympathetic surge acts directly upon cell surface receptors, which stimulate
cyclic-AMP-mediated lipoprotein lipase. Thyroid hormone also surges at birth and augments this effect (5,25,26). β-Oxidation is uncoupled in brown adipocytes, however, resulting in triglyceride breakdown and resynthesis producing heat (27).
cyclic-AMP-mediated lipoprotein lipase. Thyroid hormone also surges at birth and augments this effect (5,25,26). β-Oxidation is uncoupled in brown adipocytes, however, resulting in triglyceride breakdown and resynthesis producing heat (27).
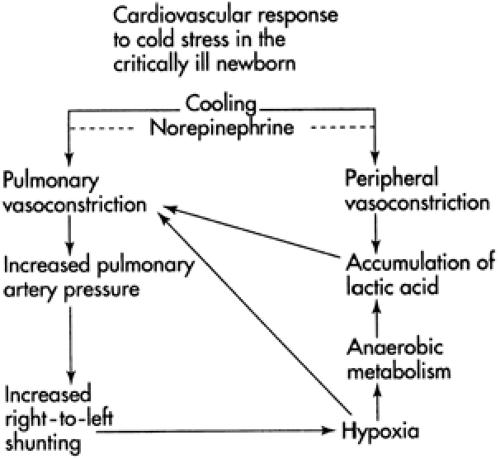
Preterm infants with immature thermogenic response and without metabolic substrate reserves deposited over the last trimester of pregnancy fare worse. As shown in Fig. 24-3, a sympathetic surge occurs at birth, with massive neurohumoral secretion of noradrenaline from paraaortic nodes and the fetal adrenal (28). Systemic and pulmonary vasoconstriction result, which may end in poor oxygen uptake and relative peripheral tissue hypoxia. Lactic acid production ensues, with demise ultimately occurring secondary to cold stress. Tarnier in the 1800s recognized this in Paris, where there was no central heating and poor home and hospital insulation.
Early Intervention
Drying infants in the delivery suite interrupts the process of evaporation, and bundling infants in cotton blankets to prevent exposure to cold air interrupts convective heat loss and provides insulation to retain the infant’s metabolic heat. Placing infants at the mother’s breast and cradled into her axillary fold engenders conductive heat transfer from the mother to the infant. Alternatively, and especially if early intervention is required to aid transition (e.g., suctioning or oxygen administration), the infant is dried first and then placed onto dry bedding under a radiant warmer while these procedures are performed. A convectively warmed incubator enclosure with air temperatures ranging from 35.0°C to 37.0°C and a variety of plastic swaddling heat shields have been advocated to prevent excessive cold exposure, especially during transition and hospital transport of premature infants (29,30,31).
Once in the nursery or mother’s room, bundled infants may be placed into either an open bassinet or incubator (naked or bundled) and provided close monitoring of either axillary (preferred, 36.0°C to 36.5°C) or rectal (37°C to 37.5°C) temperatures through the first few hours of life. Slightly premature infants (32 to 35 weeks) or small-for-gestational-age babies may appear to have normal body temperature at the expense of metabolically generated heat (9). Glass and associates (32) demonstrated that premature infants nurtured in dry incubator environments of either 35.0°C (slightly cool) or 36.5°C in the first few days of life maintained a normal body temperature, but experienced more weight loss in the cooler environment.
Kangaroo Care
Skin-to-skin care, now termed kangaroo care, has been promoted for nurturing premature infants who are held naked between the mother’s breasts as if in a kangaroo’s pouch. The infant is in contact with the mother’s warm skin and is close to the breast for unlimited feeding. Fathers also can provide thermal support in this way. Kangaroo care was first reported from Bogotá, Columbia, where use of conventional incubators was limited and mortality in nonincubated preterm births high. A large randomized trial from this country recently showed that infants less than or equal to 2.00 kg placed under kangaroo care shortly after birth for prolonged periods achieved transition safely and grew normally, had fewer nosocomial infections, and were discharged earlier, particularly at less than or equal to 1.80 kg (33). Significant reduction in early mortality also has been observed. During the 1980s the kangaroo technique was promoted for nurturance of nonmechanically ventilated, growing premature infants in Scandinavian and some other European countries. Randomized clinical trials also have demonstrated enhanced mother-infant attachment, greater maternal self-esteem, prolonged and enhanced lactation, increased infant alertness, and better weight gain (34). Physiologic studies have focused on demonstrating thermal-neutral metabolic response (minimal observed oxygen consumption) and temperature stability in stable growing premature babies during kangaroo care. Moreover, vital signs and oxygenation parameters were demonstrated to be more stable in preterm infants recovering from bronchopulmonary dysplasia, with absence of periodic breathing and reduced apnea and bradycardia. Behavioral studies demonstrate more homogenous sleep patterns, less irritability later in infancy, and more direct social eye contact with caregivers (35).
In the intensive care nursery, kangaroo care may be initiated even during mechanical ventilation with uncomplicated patients. Mothers are instructed to wear front-opening shirts, maintain careful hygiene without open sores or rashes, and avoid use of lotions, oils, or perfumes. Maximum skin surface area contact is desirable with a covering blanket to avoid outward convective and evaporative heat losses. Privacy and quiet must be provided by the nursery staff for periods of 0.5 to 1 hour initially, and careful temperature monitoring by surface thermistor or axillary thermometer should be performed at least every 15 minutes,
along with cardiorespiratory and noninvasive oxygen monitoring when indicated. Temperature deviation of more than 0.5°C of normal skin temperature (36.5°C to 37.0°C) should result in termination of the session and return to incubator care. Periods up to 4 hours may be achieved. Mothers are encouraged to pump their breasts before and after sessions because milk production is enhanced. Parents are empowered with the care of their infants, and kangaroo care integrates the family into the neonatal intensive care team. Presently, no adverse reports have been published, and the use of kangaroo care in modern intensive care settings is on the rise.
along with cardiorespiratory and noninvasive oxygen monitoring when indicated. Temperature deviation of more than 0.5°C of normal skin temperature (36.5°C to 37.0°C) should result in termination of the session and return to incubator care. Periods up to 4 hours may be achieved. Mothers are encouraged to pump their breasts before and after sessions because milk production is enhanced. Parents are empowered with the care of their infants, and kangaroo care integrates the family into the neonatal intensive care team. Presently, no adverse reports have been published, and the use of kangaroo care in modern intensive care settings is on the rise.
CONVECTION-WARMED INCUBATORS
A modern incubator consists of an optically transparent, plastic hood (≥3 mm thick) covering the infant, with sidewall and hand-access ports. The infant lays on a bed platform, underneath which a tungsten element electronically heats the air. Air is forced over this element by a fan, circulating heated air within the hood. Temperature may be controlled thermostatically to regulate either the air or infant skin temperature (15,36).
Thermodynamics of Incubation
The physiology of mammalian (homeothermic) thermal regulation (37) may be summarized by the equation:
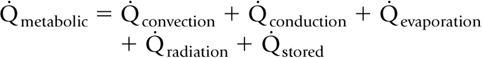
in which [Q with dot above] is the rate of either metabolic heat production (left side of the equation) or of heat loss and heat stored (right side of the equation), generally expressed in kcal/kg/hour or in W/m2 (J/sec/m2). By convention, heat production and heat losses (or storage) are expressed as positive values. When a mammal is successfully maintaining normal body temperature, heat storage is zero, otherwise body temperature either increases or decreases until a new thermal equilibrium is established at another temperature. Also, when an environmental heat loss becomes a heat gain (e.g., under a radiant warmer), the gain is ex-pressed as a negative loss.
Convection
The rate of heat transferred from an infant’s skin into the incubator environment depends in part on the insulation provided by the dermis and subcutaneous fascia (comprised primarily of white fat deposited late in gestation). Preterm babies have almost no fatty fascia and, therefore, are more vulnerable to heat loss through air (and skin blood flow) convection (37). Air convection is heat loss that takes place from the skin’s surface into the surrounding environment and is summarized by the equation:
in which two forms of skin-to-air convective heat loss occur, depending on the gradient between skin and air temperatures (ΔT), the complex geometry of surface area exposed and air thermal density k, and air movement velocity Vn (38). The first is natural convection, which results from the gradient of temperature between the skin surface and surrounding air (38,39). Natural convection cells form as warm air rises from the skin, conveying heat and body moisture away from the surface of the baby. Air thus warmed subsequently cools and falls back toward the baby, forming the convection cell. Such cells form over the curvature of the baby’s exposed body surface area. An infant in flexion leaves less surface area exposed (38). An infant extended and flaccid is able to dissipate more heat. Posture may be a valuable observation in deciding the thermal comfort or discomfort of even a preterm infant.
The second form of convection is forced convective air movement, usually occurring at air velocities ≥0.27 m/sec. Forced convective heat loss is roughly proportional to an exponential power (n) of the velocity (V) of air movement. Within forced convection-warmed incubators, manufacturers strive to render still the air near the baby. Recent estimates of natural and forced-air convective heat loss from premature neonates nursed within incubators indicate success in this strategy because natural convection was the only major loss observed to occur (40). Heat also is lost to a lesser degree by respiratory convection and evaporation.
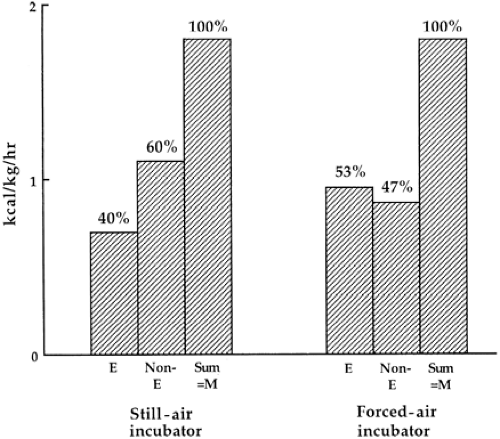
An example of different incubator convection designs affecting skin-to-air heat transfer is shown in Fig. 24-4,
adapted from a study of partitional calorimetry performed by Okken and associates (41). The left incubator partition in this figure represents a natural convection-warmed incubator with no circulating fan. Air rose passively from heating elements underneath the baby’s mattress to warm the interior of the incubator hood. The second partition on the right represents a more standard, fan-forced convection incubator as described previously. Nonevaporative heat loss in the passive device (non-E, the sum of convection, radiation, and conduction) was 60% compared to 47% in the forced convection-warmed incubator, whereas evaporative heat loss (E) was 13% higher in the forced convective environment. These authors attributed this increased evaporative loss to disturbance by the incubator’s fan of a microenvironment of humid air layered near the baby’s skin.
adapted from a study of partitional calorimetry performed by Okken and associates (41). The left incubator partition in this figure represents a natural convection-warmed incubator with no circulating fan. Air rose passively from heating elements underneath the baby’s mattress to warm the interior of the incubator hood. The second partition on the right represents a more standard, fan-forced convection incubator as described previously. Nonevaporative heat loss in the passive device (non-E, the sum of convection, radiation, and conduction) was 60% compared to 47% in the forced convection-warmed incubator, whereas evaporative heat loss (E) was 13% higher in the forced convective environment. These authors attributed this increased evaporative loss to disturbance by the incubator’s fan of a microenvironment of humid air layered near the baby’s skin.
TABLE 24-1 CALCULATED BODY SURFACE AREA: BODY MASS RATIO FOR ADULTS, LOW-BIRTH-WEIGHT NEONATES, AND VERY-LOW-BIRTH-WEIGHT NEONATES | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
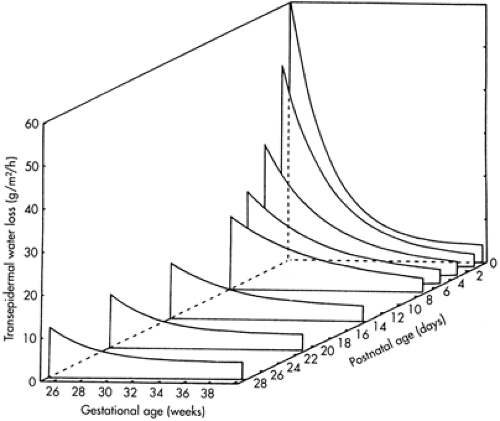 Figure 24-5 Transepidermal water evaporation from the skin of premature neonates of gestations ranging from 25 to 40 weeks, followed longitudinally from birth over the first month of life. Dehydration is most dangerous in the most immature babies less than 28 weeks of gestation in the first week of life before skin keratinization occurs. (From Sedin G, Hammarlund K, Nilsson GE, et al. Measurements of transepidermal water loss in newborn infants. Clin Perinatol 1985;12: 79-99, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|