The Otolaryngologist Approach to Obstructive Sleep Apnea
Introduction
Pediatric obstructive sleep apnea (OSA) often presents in the pediatric population with loud snoring, respiratory pauses and mouth-breathing. OSA is caused by anatomic variations and is complicated by the diminished pharyngeal muscle tone and neurophysiologic changes that typically accompany sleep. Tonsil and adenoid hyperplasia is the most common cause, but craniofacial abnormalities, palate, base of tongue, supraglottic, and glottis may also be contributory sites. Successful management in children depends on the accurate identification of the site of obstruction and the assessment of the severity, with polysomnogram as an important adjunct. This enables decision-making for the appropriate surgical and non-surgical interventions.1
Evaluation of the Patient with OSA
History
Snoring is the primary complaint of children presenting to the otolaryngologist; the prevalence has been reported as 3–12% in children,2 although some studies suggest that the rate may be as high as 27%.3 Primary snoring has implications with associated morbidity of elevated blood pressure and reduced arterial distensibility.4 Approximately 40% of children who snore have more significant manifestations with obstructive sleep apnea (OSA). Additional complaints may include mouth-breathing, breath-holding, and gasping.5 Sleep is disrupted by episodic arousals resulting from partial obstruction of the upper airway. Manifestations include morning headache, dry mouth, halitosis, and behavioral and neurocognitive disorders less commonly sleepwalking, parasomnoliloquy, enuresis, night terrors, or bruxism.5,6 Dysphagia may occur with significant tonsil enlargement. Children may avoid certain foods that are more difficult to swallow. Major predisposing factors identified in upper airway obstruction include: anatomic narrowing, abnormal mechanical linkage between airway dilating muscles and airway walls, muscle weakness, and abnormal neural regulation.7 Complications related to severe OSA include cor pulmonale, right ventricular hypertrophy, congestive heart failure, hypoventilation, pulmonary hypertension, pulmonary edema, and failure to thrive.8 History alone is insufficient to diagnose OSA. Symptoms often worsen during REM sleep when parental observation is absent.9 A polysomnogram (PSG) is the gold standard in differentiating between the spectrum of snoring, increased upper airway resistance, and OSA.
Infants with upper airway obstruction can present with stridor. This worsens with activity or feeding. Inspiratory or biphasic stridor may indicate laryngomalacia, vocal fold dysfunction (VCD), or supraglottic lesions.10 Biphasic or expiratory stridor may indicate VDC, a lower airway lesion such as subglottic stenosis or tracheobronchomalacia.10 Concerning symptoms include retractions observed apneic episodes, cyanosis, and failure to thrive. Previous history of intubation and prematurity are specific risk factors for obstructive airway lesions.11
Other factors may place a child at risk (Table 33.1). Obesity is a risk factor that may cause fatty infiltration of the pharyngeal soft tissues, which narrows the upper airway and contributes to airway resistance and reduced lung volume.12,13 The prevalence of OSA in children with Down syndrome ranges between 31% and 100%,14 and may be related to hypotonia, relative macroglossia, midfacial and mandibular hypoplasia, a narrow nasopharynx, and a shortened (high-arched) palate.15 Children with other craniofacial syndromes and abnormalities also have a higher prevalence of OSA. Robison and Otteson16 found that 172 out of 459 (37.5%) children with nonsyndromic cleft palate had symptoms of OSA, and that 39 (8.5%) had a positive PSG. Children with Apert, Crouzon, Nager, and Pfeiffer syndromes have a high rate of OSA.17,18 Other medical conditions with a high risk for the development of OSA include mucopolysaccharide disease, vascular malformations of the head and neck, and children with neuromuscular disorders.
Table 33.1
Risk factors for Sleep Disordered Breathing
| Anatomic factors | Lingual tonsil hypertrophy | Adenotonsillar hypertrophy |
| Airway lesions: | Laryngomalacia | |
| Tracheobronchomalacia | ||
| Vocal fold paralysis | ||
| Subglottic stenosis | ||
| Craniofacial abnormalities: | Down syndrome | |
| Pierre–Robin sequence | ||
| Apert | ||
| Crouzon | ||
| Pfeiffer | ||
| Hallerman-Strief | ||
| Vascular anomalies | ||
| Systemic factors | Obesity | |
| Neurologic impairment | ||
| Mucopolysaccharide diseases |
OSA may exacerbate underlying medical conditions such as congenital heart disease, pulmonary disease, and sickle cell disease (SCD). In SCD, adenotonsillar hypertrophy or recurrent tonsillitis is frequently linked with an increased risk of OSA, cerebrovascular ischemia, or frequent pain episodes, and often require an adenoidectomy and/or tonsillectomy.19 Adenotonsillectomy has been demonstrated to be cost-effective for treating obstructive sleep apnea and preventing cerebrovascular ischemia without increasing vaso-occlusive pain episodes or long-term acute service costs in routine clinical practice settings.
Physical Examination
Oral cavity examination should include evaluation of the tonsils, tongue and palate. Tonsil grading is typically on a scale of 0 (surgically absent) to 4 (tonsils touching each other) (Figure 33-1). The static pressure and/or area relationships of the passive pharynx were endoscopically measured in 14 children with obstructive sleep apnea and in 13 healthy children under general anesthesia with complete paralysis; it was determined that children with obstructive sleep apnea closed their airways at the level of enlarged adenoids and tonsils at low positive pressures, whereas healthy children required subatmospheric pressures to induce upper airway closure.20 The cross-sectional area of the narrowest segment was significantly smaller in children with obstructive sleep apnea and particularly in the retropalatal and retroglossal segments. Thus, both congenital and acquired anatomic factors clearly play a significant role in the pathogenesis of pediatric obstructive sleep apnea. Macroglossia, such as in the case of Down syndrome or Beckwith–Wiedermann, may be contributory to upper airway obstruction. The appearance of the palate should be noted, as an elongated palate may also play an important role in obstruction, particularly after adenotonsillectomy (Figure 33-2).
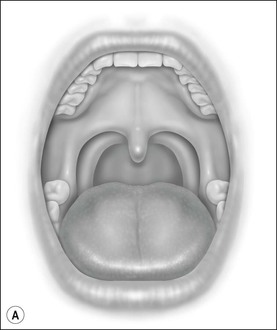
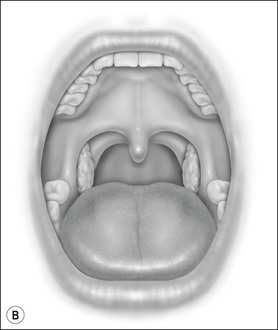
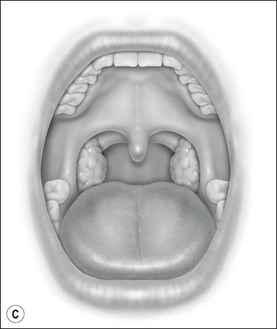
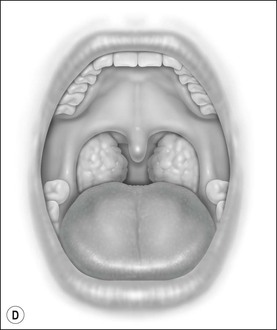
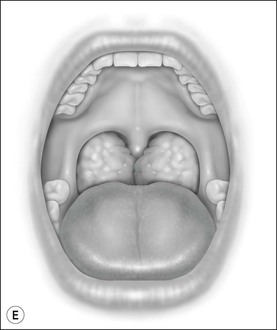
Figure 33-1 Tonsil Staging. Friedman M. Friedman tongue position and the staging of obstructive sleep apnea/hypopnea syndrome. In: Friedman, editor. Sleep Apnea and Snoring. Surgical and Nonsurgical Therapy. China: Saunders, Elsevier; 2009. Fig 16.2, p 108.
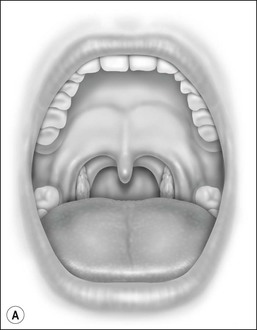
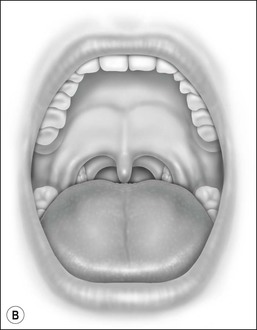
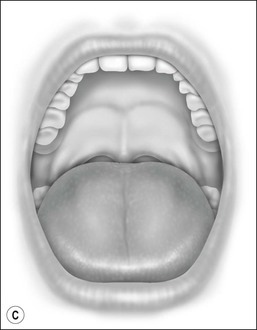
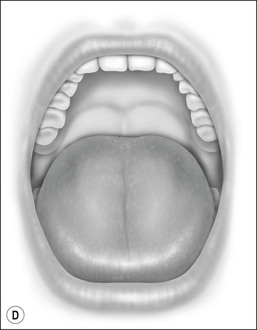
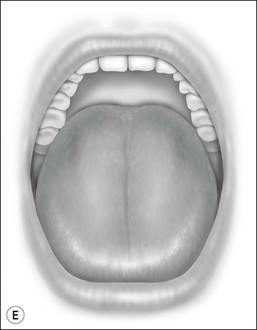
Figure 33-2 Friedman’s Classification of Palate Position. Friedman M. Friedman tongue position and the staging of obstructive sleep apnea/hypopnea syndrome. In: Friedman, editor. Sleep Apnea and Snoring. Surgical and Nonsurgical Therapy. China: Saunders, Elsevier; 2009. Fig 16.1, p 106.
Flexible endoscopy is an important tool that can allow for comprehensive evaluation of the upper airway in infants and children. This may be performed in the office in an awake child or with anesthesia. Lidocaine is used for topical anesthesia and oxymetazolone for decongestion to increase the comfort for this examination. Sites of obstruction may occur anywhere from the tip of the nose to the glottis. Therefore endoscopy can be used to visualize the nasal passages, nasopharynx, oropharynx, hypopharynx, and larynx. Nasal patency is assessed for nasal septal deviation, turbinate hypertrophy, polyps, or masses. Choanae are evaluated for stenosis or atresia. Adenoids may be visualized for hypertrophy. Oropharyngeal and hypopharyngeal examination provides a view of the posterior aspect of the tonsils and base of tongue. Tonsils may appear normal during an oral cavity exam. However, the tonsil pole may be elongated or posteriorly displaced and occupy a significant portion of the hypopharynx. Base of tongue enlargement may result in effacement of the vallecular with compression and displacement of the epiglottis posteriorly. Enlargement of the lingual tonsils at the tongue base is increasingly recognized as a treatable cause of OSA. Obese children have a high frequency of enlargement of the lingual tonsils with a significantly higher prevalence in those with previous tonsillectomy,21 as demonstrated by MRI. Flexible endoscopy with the child in a supine position may further highlight narrowing at the level of the hypopharynx. Collapse of the lateral pharyngeal walls during inspiration with a closed airway (mouth shut and nostrils occluded) may be noted. The supraglottic structures are evaluated for laryngomalacia, as identified with epiglottis and/or arytenoids prolapse over the glottis. The vocal folds are assessed for mobility and masses.
Polysomnogram
Although history, physical examination, and flexible endoscopy of the airway are important tools, these cannot fully simulate the complexity of airway dynamics during sleep. Polysomnogram (PSG) is a valuable asset in assessing breathing. Indications for PSG have been outlined in the Clinical Practice Guideline developed by the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS).22 Children with complex medical conditions such as obesity, Down syndrome, craniofacial abnormalities, neuromuscular disorders, sickle cell disease, or mucopolysaccharidoses may benefit from preoperative PSG. PSG may be indicated in children for whom the need for surgery is uncertain, such as in the case of tonsillar hypertrophy and lack of sufficient history or when significant symptoms are present in the face of a negative physical examination.22 Prior to surgical intervention and anesthesia induction, the results of the study should be relayed to the anesthesia team. Inpatient admission after surgery should be considered for children under the age of 3 years or with severe OSA by PSG (apnea–hypopnea index of 10 or more obstructive events/hour, oxygen saturation nadir less than 80%, or both).23,24 Intensive care unit monitoring may be warranted in a child with very severe OSA, medical comorbidities that cannot be managed on the floor, and those who demonstrate significant airway obstruction and desaturation in the initial postoperative period that requires interventions beyond repositioning and/or oxygen supplementation.22
Imaging
Plain radiography with a high-kilovoltage lateral neck film may be used to evaluate for adenotonsillar hypertrophy in a child who is difficult to examine or when a caregiver defers flexible endoscopy. Adenoid hypertrophy visualized by lateral neck radiograph correlates well with flexible nasopharyngoscopy.25 Most techniques focus on the size of the nasopharyngeal stripe, which indicates the amount of airflow through the nasopharynx. When the nasopharyngeal stripe is half the size of the soft palate, significant obstruction occurs. However, the actual size of the adenoid pad is not as relevant as the amount of obstruction caused by hypertrophy.
Anterior–posterior high-kilovoltage neck films evaluate the laryngeal suprastructure and subglottis. Cephalometry and computed tomography are useful in evaluating children with craniofacial disorders. Research suggest that there are evident and early changes in facial growth and development among children with OSA, characterized by increased total and inferior anterior heights of the face, as well as a more anterior and inferior position of the hyoid bone.26 Chest radiography may help identify evidence of pulmonary hypertension or right ventricular hypertrophy in a child with severe OSA.27
Cine magnetic resonance imaging is a useful radiographic adjunct to the physical examination because it allows the clinician to screen for and to observe airway collapse in three planes (axial, coronal and sagittal).28,29 This is particularly helpful in isolating anatomic sites of airway obstruction in children who have persistent apnea after adenotonsillectomy (Tonsillectomy and Adenoidectomy).
Additional Testing
Patients with severe OSA or abnormal electrocardiogram findings on PSG warrant a complete electrocardiogram. Electrocardiographic findings are not specific for pulmonary hypertension or right ventricular hypertrophy but may include voltage or axis abnormalities, a t-wave pattern (strain pattern), or mixed criteria. On occasion, a normal electrocardiogram may occur even with echocardiographic findings of pulmonary hypertension.27 Laboratories are not routinely ordered prior to Tonsillectomy and Adenoidectomy unless a history is concerning for a bleeding disorder.30 Patients who have a history of easy bruising and prolonged bleeding warrant a CBC/PT/T. Arterial blood gas analysis in conjunction with PSG may demonstrate hypoxemia or hypercarbia. Chronic hypercarbia may be associated with a compensatory metabolic alkalosis.27
Medical Intervention
Antibiotics are not beneficial in long-term reduction of tonsil hypertrophy, although during acute inflammation broad-spectrum antibiotics may provide a short-term decrease in tonsil size; one study reported only 15% of patients avoided surgery in long-term follow-up.31
Intranasal Steroids
Intranasal steroids may be helpful if adenoid hypertrophy is the predominant cause of mild OSA in children. Decreases of 29% in adenoid size and 82% in symptom scores were noted in one study.32 Another study assessed a 6-week course of intranasal steroids,33 reporting a significant improvement of the AHI post-treatment compared to the placebo group. Results may be maintained 8 weeks after completion of active treatment.34 Intranasal steroids have not been found to impact tonsil size.33
Twenty-four children with residual OSA (AHI >1 and <5/hour) were treated with montelukast and intranasal budesonide aqueous solution for 12 weeks.35 This combined anti-inflammatory therapy effectively improved and/or normalized respiratory and sleep disturbances in children with residual SDB after adenotonsillectomy.
Systemic steroids have been tried in non-acute adenotonsillar hypertrophy. A short-term decrease in tonsil size has been noted, but long-term improvement and avoidance of surgery are usually not seen.36 Although there is indication that intranasal steroids and monteleukast may be helpful in mild OSA, pharmacologic agents have no significant and persistent effect in the treatment of moderate to severe OSA in children.
Oral Appliances
Villa et al. randomized children with OSA and dysgnathia to either a personalized jaw-positioning oral appliance for 6 months or no intervention.37 The authors reported that the AHI in the intervention group decreased significantly from 7.1 to 2.6/h. The oral appliance significantly lowered parent-reported snoring and apnea. A few of the patients had excessive salivation that subsided spontaneously. A Cochrane review (2007) on oral appliances and functional orthopedic appliances for OSA in children 15 years old or younger reported that there is insufficient evidence to state that oral appliances or functional orthopedic appliances are effective in the treatment of OSA in the majority of children. However, they may be helpful in the treatment of children with craniofacial anomalies that are risk factors of apnea.38
Adenotonsillectomy
If OSA is present, tonsillectomy alone or adenoidectomy alone is not sufficient, and adenotonsillectomy should be performed together. Fortunately, because most pediatric OSA is caused by adenotonsillar hypertrophy, T&A alone is an effective and durable treatment for most children39 and is the first choice for children with OSA.40–42 There is an indication that children older than 3 years of age, in the absence of comorbidities, can safely undergo adenotonsillectomy without undergoing preoperative PSG, provided those patients have close postoperative monitoring.43
The preoperative evaluation of children scheduled for adenotonsillectomy should include careful assessment for evidence of upper airway obstruction, pulmonary hypertension, and right heart dysfunction. The anesthesiologist will screen for symptoms of upper airway obstruction and congenital or acquired disorders associated with abnormal facial structure, decreased muscle tone or development, developmental delay and/or cardiopulmonary disease.44 Other clinical findings in patients with pulmonary hypertension and right ventricular dysfunction can include jugular venous distension, hepatomegaly, loud pulmonic component of the second heart sound, right ventricular heave and peripheral edema, failure to thrive, developmental delay, and altered mental status.44
T&A may be performed safely in an outpatient setting in appropriate selected patients. The AAO-HNS society recommends that patients be greater than 3 years of age, not have OSA, and be American Society for Anesthesia (ASA) Class I/II (low anesthesia risk).45 Parents must be advised that a low threshold for readmission exists.45 The postoperative complication rate is higher in children with craniofacial disorders, failure to thrive, neurological impairment, Down syndrome, obstructive sleep apnea, and in children age 3 years or less.24 Children who are at risk for perioperative complications should remain overnight for observation.
The most common complications of adenotonsillectomy are anesthesia risks, pain, otalgia, and bleeding.46 Dehydration may occur due to poorly controlled pain, refusal of oral intake, nausea and vomiting secondary to narcotic use.46 Rare complications of tonsillectomy include subcutaneous emphysema, pneumomediastinum, and taste disturbance due to damage to the lingual branch of the glossopharygngeal nerve.47,48 Nasopharyngeal stenosis, also an uncommon outcome, results from approximation of raw mucosal surfaces during the healing process.46
Multiple techniques and tools may be used to perform tonsillectomy. The literature presents a variety of evidence regarding outcomes related to device usage, but consensus has not been reached regarding the optimal technique with the lowest morbidity.49,50 Device choice may be influenced by exposure in training programs as well as operator experience. Patient recovery times, postoperative morbidities, as well as cost related to the device are also important factors that may drive device choice.
The most common techniques used include cold steel dissection and electrosurgical dissection. Cold steel dissection is the oldest proven technique, and the basic approach has not changed significantly in the past 100 years.51 Electrocautery is typically used for hemostasis. Electrosurgical dissection uses thermal energy to dissect tissues with either a monopolar or bipolar tip; little blood loss occurs with this technique.52 Postoperative morbidities for these techniques are similar, and include hemorrhage (1.2–2.1%), dysphagia, and otalgia.52
Plasma surgical dissection is a technique that ablates and coagulates soft tissue by generating a field of ionized sodium molecules. Many studies have shown that plasma surgical dissection tonsillectomy causes less pain, has a shorter recovery period and requires less postoperative narcotics than other methods of tonsillectomy.53
The Harmonic scalpel (vessel sealing system) uses ultrasonic energy to vibrate its blade at 55 kHz, providing simultaneous cutting and coagulation of the tissue with less trauma.54 Postoperative hemorrhage and intraoperative blood loss are low. Some studies have variably found decreased postoperative pain compared to electrosurgical dissection.54
Adenoidectomy may also be performed with a variety of devices, with either a transnasal or transoral approach. Revision adenoidectomy occurs at a rate of 1.3%.55 Reasons for revision include persistent symptoms ranging from adenoiditis to recurrent otitis to OSA. Device choice for T&A is driven by surgeon experience and attempts to decrease postoperative morbidity.
Partial tonsillectomy, also known as tonsillotomy or intracapsular tonsillectomy, may be performed for tonsil hypertrophy with UAO, but is contraindicated for chronic tonsillitis. The premise of partial tonsillectomy is reduction of obstructive tonsillar tissue while sparing the tonsillar capsule, thus preventing exposure of the underlying pharyngeal muscles. Technology utilized for partial tonsillectomy includes microdebrider techniques or radiofrequency devices. Eviatar et al.,56 in a small retrospective study of partial tonsillectomy compared to complete tonsillectomy, observed no difference in all parameters compared: non-obstructing tonsils recurred (97% vs 87%); snoring (3% vs 12.5%); recurrent tonsillitis (6% vs 6.25%); and recurrent obstruction and unilateral enlargement (3% vs 12.5%) at 8 to 10 years. An advantage of partial tonsillectomy includes decreased hemorrhage rates; however, intraoperative bleeding is increased, and may impede visualization at times.57
Success rates have been reported for T&A ranging from 59.8% to 85%, with a significant improvement in AHI from preoperative levels41 as well as an overall improvement in quality of life.58–60 However, the correlation between improvements in respiratory parameters and improvements in quality of life is poor.39 Mitchell39 evaluated outcomes in a prospective cohort study of children (ages 3–14, mean 6.3, included 79 healthy children, 40 of who were male) undergoing T&A for obstructive sleep apnea in children. Measures included PSG and OSA-18. Patients with an AHI of 5 or greater underwent adenotonsillectomy. OSA was classified as mild (AHI ≥ 5 < 10), moderate (AHI ≥ 10 < 20), or severe (AHI ≥ 20). For all children, the preoperative AHI value was higher than the postoperative value. The mean preoperative AHI for the study population was 27.5, whereas the mean postoperative AHI was 3.5 (P < 0.001). The percentage of children with normal polysomnography parameters after adenotonsillectomy ranged from 71% to 90%. Overnight respiratory parameters after adenotonsillectomy were normal for all children with mild OSA. Three (12%) children with moderate preoperative OSA, and 13 (36%) children with severe preoperative OSA had persistent OSA after adenotonsillectomy. Resolution of OSA occurred in all children with a preoperative AHI ≤ 10 and in 73% of children with a preoperative AHI < 10. The mean total OSA-18 score and the mean scores for all domains showed significant improvement after surgery (P < 0.001). The preoperative AHI values had a fair correlation with the preoperative total OSA-18 scores (r = 0.28), but postoperative AHI values had a poor correlation with the postoperative total OSA-18 scores (r = 0.16). Caregivers reported snoring some, most, or all of the time in 22 (28%) children; this group included all children with persistent OSA. T&A for OSA results in a dramatic improvement in respiratory parameters and the quality of life in the majority of healthy children with OSA.
However, children with severe preoperative OSA, asthma, age greater than 7, or obesity are at risk for persistence of OSA after T&A.61–63 T&A outcomes may not be favorable in patients with severe preoperative OSA or when obesity is present;64,65 however, it remains the currently recommended initial treatment for OSA in obese patients. Increasing rates of residual obstructive sleep apnea indicate a future role for adjunct medical as well as surgical interventions. Postoperative polysomnograms are recommended 6–8 weeks following surgery for those with additional risk factors for OSA: age < 3 years, severe OSA present on pre-perative PSG (a respiratory disturbance index of 19 or greater), cardiac complications of OSA (e.g., right ventricular hypertrophy), failure to thrive, obesity, prematurity, recent respiratory infection, craniofacial anomalies, neuromuscular disorders, or those with a high apnea index to ensure that OSA is resolved.66 Patients with mild to moderate OSA who have complete resolution of signs and symptoms require follow-up clinical evaluations but do not require repeat PSG.67 Postoperative reports of symptoms such as snoring and witnessed apneas correlate well with persistence of OSA after T&A.39



