), which imply a proportionally large pulmonary ventilation (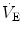 ), achieved mostly by breathing at high rates. Indeed, fast breathing is a common characteristic of all newborn mammals. With respect to maturation, the development of the lung and respiratory apparatus is never completed at birth, even in the most precocial species. The lungs continue to grow postnatally, in a centripetal direction, with formation of peripheral airways and alveoli, implying that in newborns the central airways comprise a larger proportion of the total air space and contribute to a relatively large anatomical dead space. The incomplete myelinization and low conduction velocity of neural fibers, including those of the laryngeal, vagi, and carotid sinus nerves, limit the afferent sensory information involved in the regulation of breathing. Finally, with respect to the adaptation to air breathing at birth, a multitude of events among the most dramatic of the whole life occur quite rapidly, such as the transition from filtration to absorption of the pulmonary fluid, the drastic changes in pulmonary circulation, and the rise in oxygenation with its implications on the function of the chemoreceptors.
), achieved mostly by breathing at high rates. Indeed, fast breathing is a common characteristic of all newborn mammals. With respect to maturation, the development of the lung and respiratory apparatus is never completed at birth, even in the most precocial species. The lungs continue to grow postnatally, in a centripetal direction, with formation of peripheral airways and alveoli, implying that in newborns the central airways comprise a larger proportion of the total air space and contribute to a relatively large anatomical dead space. The incomplete myelinization and low conduction velocity of neural fibers, including those of the laryngeal, vagi, and carotid sinus nerves, limit the afferent sensory information involved in the regulation of breathing. Finally, with respect to the adaptation to air breathing at birth, a multitude of events among the most dramatic of the whole life occur quite rapidly, such as the transition from filtration to absorption of the pulmonary fluid, the drastic changes in pulmonary circulation, and the rise in oxygenation with its implications on the function of the chemoreceptors.
This chapter touches on the general principles of operation of the neonatal respiratory system examined as a neuromechanical unit that operates to generate the optimal
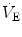
responses by integrating peripheral information with metabolic requirements. The general principles of operation of the neuromechanical unit (that is, translation of neural output into mechanical events, evaluation of the adequacy of
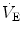
from neurochemical feedback) are the same throughout the whole postnatal life. However, some functional aspects are peculiar to the neonatal period. The goal of this chapter is to highlight the functional properties characteristics of the newborn. References are not extensive, with preference given to review articles where the interested reader can trace the background information and technical details. A short list of terminology and abbreviations adopted in the text is given in Table
2.1.
Table. 2.1
Terminology, abbreviations adopted in text, common units
Cw |
Chest wall compliance |
ΔVolume/ΔPressure |
CL |
Lung compliance |
ΔVolume/ΔPressure |
f |
Breathing frequency |
Breaths/min |
FRC |
Functional residual capacity |
ml |
P |
Pressure |
cm H2O or mmHg |
Pab |
Abdominal pressure |
cm H2O |
PaO2 |
Arterial pressure of oxygen |
mmHg |
PACO2 |
Alveolar pressure of carbon dioxide |
mmHg |
Pdi |
Transdiaphragmatic pressure |
cm H2O |
PEEP |
Positive end-expiratory pressure |
cm H2O |
Ppl |
Pleural pressure |
cm H2O |
T |
Tension |
dyne/cm |
T E |
Expiratory time |
s |
T I |
Inspiratory time |
s |
V |
Volume |
ml |
|
Flow |
ml/s |
|
Alveolar ventilation |
ml/min |
|
Pulmonary ventilation |
ml/min |
|
Oxygen consumption |
ml/min |
|
Carbon dioxide production |
ml/min |
V r |
Resting volume of the respiratory system |
ml |
V T |
Tidal volume |
ml |
W |
Weight |
kg |
2.2 Neural Output
The mechanisms involved in the generation of the neural output responsible for the breathing rhythm are still largely unexplained. Several approaches have attempted to unveil the origin of respiratory rhythmogenesis with reduced preparations, such as brainstem with or without the spinal cord or brainstem slices, commonly from neonatal rats. In vitro, it appears that a respiratory rhythm can be generated in very discrete brainstem regions (Haddad
2003), and the maturation of the putative respiratory centers has been studied extensively (Hilaire and Duron
1999). Nevertheless, discrepancies between the in vitro neural pattern and the natural breathing pattern in vivo are common and difficult to explain (Achard et al.
2005). Partly, these differences could be due to technical issues, adequate oxygenation of the preparation, and modification or abolition of the normal afferent inputs. Partly, they could reflect a fundamental flaw in the assumption that, normally, rhythmogenesis is independent of peripheral metabolic, neural, or chemical inputs. The major obstacle for a unifying interpretation of the results regarding the development of the neonatal network is the lack of an accepted model of respiratory rhythmogenesis.
What are the mechanisms that make breathing a continuous process at birth after the intermittent pattern of the fetal period is an unsolved mystery in perinatal physiology. The number and intensity of stimuli at birth – tactile, pressure, thermal, visual, and acoustic – are large. In addition, internal afferent inputs, like those of the chemoreceptors and of the airway mechanoreceptors, change markedly because of the rapid changes in oxygenation and in transpulmonary pressure. Under controlled experimental conditions, each of the putative stimuli in isolation can be shown to be important in initiating breathing. In fact, it is reasonable to assume that any stimulus can take a dominant role in specific situations (Jansen and Chernick
1991). Arousal, oxygenation, and a general state of stress (Lagercrantz and Slotkin
1986) increase metabolic rate, which by itself (through the increase in CO
2 production) stimulates breathing. Experiments on animal fetuses have indicated that exteriorization and clamping of the chord are not sufficient to trigger and maintain continuous breathing unless also CO
2 is allowed to rise (reviewed in Mortola
2001). In birds, the trigger, increase, and maintenance of
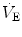
at hatching are believed to be caused by the rise of metabolically produced CO
2 (Mortola
2009). Hence, it is quite possible that the risen level in metabolic rate at birth represents the main mechanism sustaining continuous
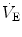
.
In newborns, the phrenic output is typically an abrupt and short burst of activity with few spikes, quite different from the activity-reach ramp-shaped firing of the adult phrenic nerve. The rapid and short-lasting neural burst leaves little room for fine modulation. Presumably, this is one reason for the fact that in newborns peripheral inputs often play only a course control on the breathing pattern, which is typically irregular and variable. In fact, in the neonatal period peripheral neural afferents, despite being less in number and with lower firing frequency than in adults, can generate quite drastic reflex effects on breathing (2.5.1).
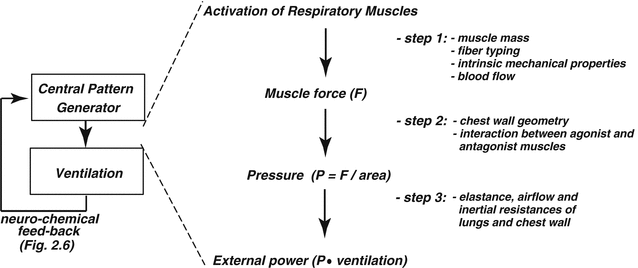
2.3 Translation of Muscle Contraction into Pulmonary Ventilation
The
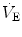
generated by the contraction of the respiratory muscle results from three key steps (Fig.
2.1). First, the intrinsic mechanical characteristics of the muscle fibers determine the force that the muscle is able to generate. Then, the force translates into pressure, the magnitude of which depends on the geometrical characteristics of the muscle and the structure over which the force is applied. Finally, the pressure generates airflow and causes changes in lung volume according to the flow-resistive and elastic properties of the respiratory system.
2.3.1 Step 1. Force Generation
For a long time it was suspected that the respiratory muscles of the newborn operate close to the fatigue threshold, as if they were barely capable to fulfill the ventilatory requirements. This view probably stemmed from the observation that breathing in neonates, especially premature infants, often is irregular and interspersed with periods of short apneas. The finding that the so-called “fatigue-resistant” muscle fibers were underrepresented fueled the idea that muscle failure was a contributor to periodic breathing and apneas (Keens et al.
1978; Le Souëf et al.
1988; Watchko et al.
1992; Vazquez et al.
1993). In reality, diaphragm fiber typing does not necessarily correlate with muscle fatigability (Sieck and Fournier
1991), and changes in diaphragmatic activity do not correlate with the infant’s periodic breathing or apneas (Nugent and Finley
1985). Studies on newborns of various species have shown the neonatal diaphragm to be at least as fatigue-resistant as the adult’s diaphragm, capable of performing well even in the face of major workloads (Lieberman et al.
1972; Maxwell et al.
1983; Powers et al.
1991). Blood perfusion of the neonatal diaphragm is at least as adequate as it is in adult (Soust et al.
1989; Berger et al.
1994), even at high contraction rates or against resistive loads.
2.3.2 Step 2. Pressure Generation
In mammals, the total mass of the respiratory muscles, in relation to body mass, is almost a fixed proportion irrespective of species and animal age. The force produced by a muscle is proportional to its cross-sectional area, and the resulting pressure is the ratio between the force produced and the surface which the force is applied on. Because of their small size, the neonatal respiratory muscles produce little force by comparison to adults (Sieck et al.
2002). However, the surface over which such force is applied is proportionally small; hence, mammals of all sizes and ages do not differ much in the pleural pressure that their respiratory muscles can generate (Mortola
2001).
The shape of the diaphragmatic dome is an additional factor in the translation of force into pressure. In fact, by application of the Young-Laplace relationship, for a given diaphragmatic tension
T, the resulting transdiaphragmatic pressure Pdi
1 depends on the radius of curvature
r of the dome (Pdi =
T/
r). In newborns, the fact that
r is much smaller than in adults favors the generation of a greater Pdi for a similar
T. In conclusion, despite the fact that muscle mass and muscle force are undoubtedly less in newborns than in adults, the tidal swings in pleural pressure (Ppl) are similar at all ages, about 5–7 cm H
2O.
2 If needed, the healthy newborn can generate much higher Ppl values, up to 100 cm H
2O, as is the case during the first inspiration (Mortola
2001). The infant’s maximal inspiratory pressures during crying (Shardonofsky et al.
1989) are not much lower than the maximal static inspiratory pressures developed by adults.
Contraction of the diaphragm raises abdominal pressure (Pab). This increase causes the outward motion of the frontal abdominal wall during inspiration and the expansion of the lower portion of the rib cage. The latter occurs because of the mechanical interdependence between the abdomen and rib cage and because Pab gets transmitted to the thoracic wall through the apposition area. The apposition area is the lowermost region of the rib cage that the diaphragmatic dome faces without interposed lungs. In infants, differently from the adult, in inspiration the expansion of the lower rib cage is small because of two reasons. First, the high abdominal compliance limits the rise in Pab during diaphragmatic contraction. Second, the rather rounded shape of the ribs and their almost perpendicular attachment to the vertebral column limit the size of the area of apposition (Allen and Gripp
2002). With growth, the area of apposition increases because of the gradual downward orientation of the ribs, presumably caused by the gravitational pull. The result of this anatomical arrangement is that in newborns the increase in Pab during diaphragmatic contraction does not contribute to the expansion of the lower ribs as much as it does in adults. Switching from the supine to the prone position stiffens the abdomen; in fact, this postural change is functionally equivalent to binding the infant’s abdomen and improves diaphragmatic efficiency (Fleming et al.
1979; Guslits et al.
1987; Laing et al.
1988; Wolfson et al.
1992) with positive effects on blood oxygenation (Numa et al.
1997). As an aside, it is interesting to note that the sudden infant death syndrome (SIDS) has a greater prevalence in the prone position (Silvestri and Weese-Mayer
2003). This implies that in the prone position the posture-related factors involved in the physiopathology of SIDS, whatever they may be, more than offset the advantage in the mechanical operation of the respiratory system.
2.3.3 Step 3. Lung and Chest Wall Mechanics
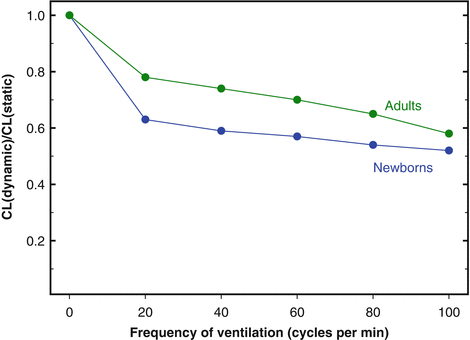
As it is the case for many other internal organs, the W-specific mass of the lung (lung weight-body weight ratio) is large in newborns and decreases during postnatal growth. Despite the large mass, air spaces are still incompletely developed, the pulmonary elastin and collagen contents are low, and during the first postnatal hours or days, some fluid is trapped in the peripheral airways and lung interstitium. All these factors contribute to values of lung compliance (CL, Δvolume/Δpressure) lower in newborns than in adults, when the comparison is made on the basis of lung volume or lung weight. The peripheral inequalities in airway resistance and the viscous properties of the pulmonary tissue prolong the response time of the lungs both in inspiration and in expiration. For these reasons, the differences between dynamic and static CL (and, possibly, the phenomenon of frequency dependence of CL
3) are more pronounced in newborns than in adults (Fig.
2.2).
The chest wall anatomically and functionally comprises two main compartments, the rib cage (or thorax) and the abdomen-diaphragm. Chest wall compliance (Cw) has been measured in newborns of many species and in infants; the results have been uniform in revealing high values (after normalization by body W) in comparison to the adult. The high Cw in newborns is a structural necessity for the passage through the birth canal. Cw decreases gradually during postnatal growth (Papastamelos et al.
1995), probably because of the stiffening of the cartilaginous structures and changes in abdominal and thoracic configuration.
Because the Cw-CL ratio is a dimensionless parameter, it can be compared directly among individuals of different age or body size, with no needs for normalization. In adult humans, because Cw and CL have similar values, their ratio is approximately equal to one. In infants, Cw is about five times higher than CL (Polgar and Weng
1979). In fact, the high Cw-CL ratio is a characteristic feature of the respiratory system of all neonatal mammals investigated (Mortola
2001). The fact that Cw is so high relative to CL implies that in infants CL is the major determinant of the compliance of the respiratory system (Crs) and that changes in Crs are an excellent indicator of changes in CL. From a practical viewpoint, this is quite convenient because measurements of CL in infancy can be difficult (due to the uncertainties in the measurements of Ppl), while measurements of Crs can be performed easily (England
1988; Wohl
1991; Mortola
2004).
2.4 Mechanical Constraints and Breathing Pattern
The high Cw-CL ratio of the newborn has two major implications. First, in inspiration, the tendency of the chest wall to distort is greater than in adults. Second, during expiration, the low ratio reduces the outward pull of the chest on the lungs and facilitates lung emptying and a low resting volume of the respiratory system (V r). These mechanical characteristics have an impact on the neonatal pattern of breathing.
2.4.1 Chest Wall Distortion
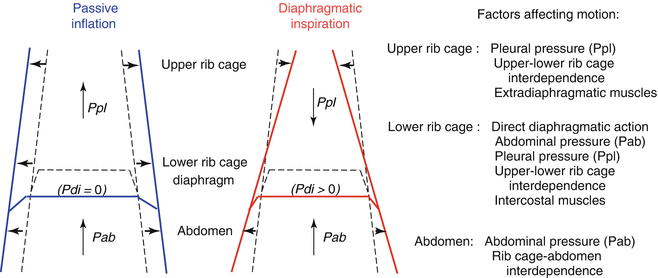
In any solid structure, distortion can be defined as a configuration that differs from that requiring minimal energy. In the case of the chest wall, distortion occurs in active conditions (i.e., during respiratory muscle contraction) when the shape deviates from the configuration assumed in the passive situation.
4 This latter is the configuration attained at any lung volume when the respiratory muscles are relaxed, as is the case, for example, during mechanical ventilation. By definition, the occurrence of distortion implies that the respiratory muscles need to spend extra energy to generate a given tidal volume (
V T). In inspiration, distortion is the unavoidable consequence of the location of the diaphragm between the two main chest wall compartments, the abdomen and thorax. In fact, during contraction (i.e., with Pdi > 0) the diaphragm operates simultaneously as a positive pressure pump for the abdomen, raising Pab, and as a negative pressure pump for the thorax, lowering Ppl. Therefore, differently from the passive inflation in which Pab and Ppl rise homogeneously and expand both abdomen and thorax, during active inspiration the abdomen expands while the rib cage caves inward (Fig.
2.3). The suction on the rib cage can be appreciated visually in tetraplegic patients, who have no control of the extradiaphragmatic muscles (Mortola and Sant’Ambrogio
1978; Thach et al.
1980). In normal conditions, the tendency to distortion is less in adults than in newborns because adults have a more rigid chest wall and greater compensatory action of the extradiaphragmatic muscles. In newborns, the highly compliant chest wall, the small area of apposition (2.3.2), and the limited mechanical linkage between lower and upper rib cage are the main factors responsible for chest distortion, of which the most obvious aspect is the paradoxical inward motion of the rib cage during inspiration and expansion in expiration. In addition, the poor activity and mechanical coordination of the intercostals muscles, especially during some phases of sleep (Muller et al.
1979), further contribute to the limited stability of the neonatal thorax.
It is difficult to estimate the energetic price of distortion. Taking the abdominal expansion as an index of diaphragmatic shortening during inspiration, in newborns during resting breathing, chest wall distortion reduces by half the inspiratory efficiency of the diaphragm (Mortola
1995). This means that, in first approximation, to achieve a given
V T, the diaphragm must contract twice as much than it would need had the system expanded along its passive configuration. An additional burden caused by chest distortion is the deformation of the lungs, which reduces CL (Sullivan and Mortola
1985) and probably worsens the ventilation-perfusion matching. As mentioned above (3.2.), the prone position, by stiffening the chest wall, reduces its distortion during inspiration and improves the mechanical efficiency of breathing.
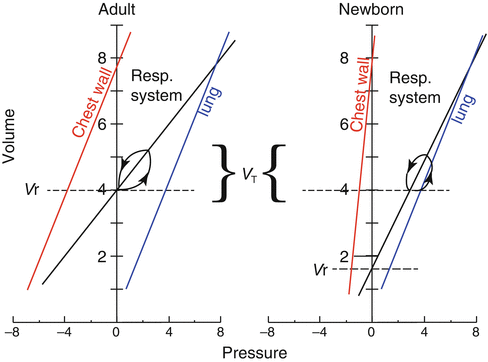
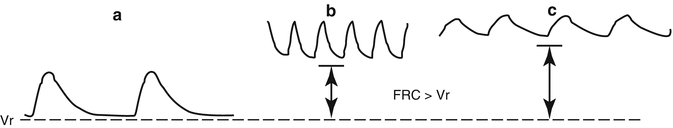
2.4.2 Low Resting Volume: Problems and Solutions
A highly compliant chest wall exerts a lesser outward pull on the lungs than a stiffer chest does, with the result that the passive resting volume of the respiratory system (
V r, the volume at which lungs and chest recoil pressures offset each other) is low (Fig.
2.4). Indeed,
V r (after normalization by lung weight) is lower in newborns than in adults of several species and in infants by comparison to the adult man (Cook et al.
1958; Fisher and Mortola
1980). The fact that
V r is low carries consequences on the functional residual capacity (FRC), which has a prominent role in the efficient operation of the mammalian respiratory system. FRC buffers the oscillations and maintains the stability of the alveolar and arterial gases and is a reserve of oxygen during occasional periods of hypoventilation or apneas. Most important is the fact that inflation of the lungs from FRC requires substantially less pressure than inflation from a collapsed state. In adults during resting breathing, FRC is essentially equal to
V r. Infants, on the contrary, compensate the potentially disadvantageous mechanical situation of the low
V r by keeping FRC dynamically elevated; hence, differently from adults, in infants FRC exceeds
V r (Fig.
2.4).
The core mechanism that permits a dynamic elevation of FRC above
V r is a mismatch between the time needed for expiratory flow (mechanical expiratory time) and the neural expiratory time (
T E), with the former being longer than the latter. In this way, inspiration begins before the air is fully exhaled, causing lung hyperinflation. At least three mechanisms operate to achieve this goal, the post-inspiratory activity of the expiratory muscles, laryngeal braking in expiration, and high breathing frequency (Fig.
2.5). The former two prolong the mechanical
T E and the latter shortens the neural
T E. All of them are operative, either together or individually, in infants (Eichenwald and Stark
2003) and in newborns of many other species (Mortola
2001). In infants, the FRC −
V r difference is 10–15 ml, or about 3 ml/kg. In cases of apnea (long neural
T E) FRC invariably decreases toward
V r. During mechanical ventilation, the presence of an endotracheal tube eliminates the newborn’s laryngeal control of expiratory flow; in this case, the application of an end-expiratory load (or positive end-expiratory pressure, PEEP) of a few cm H
2O is necessary to counteract the otherwise unavoidable drop in FRC. This becomes even more necessary in conditions of low CL because of lung disease, which further increases the Cw-CL ratio (Gregory et al.
1971; Berman et al.
1976). In fact, the FRC −
V r difference can be seen as a mechanism that generates an internal PEEP of a few cm H
2O. Although small, this positive airway pressure probably contributes to the absorption of the pulmonary fluid from the alveolar spaces into the lung interstitium during the first hours after birth (Strang
1991).
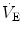 ), achieved mostly by breathing at high rates. Indeed, fast breathing is a common characteristic of all newborn mammals. With respect to maturation, the development of the lung and respiratory apparatus is never completed at birth, even in the most precocial species. The lungs continue to grow postnatally, in a centripetal direction, with formation of peripheral airways and alveoli, implying that in newborns the central airways comprise a larger proportion of the total air space and contribute to a relatively large anatomical dead space. The incomplete myelinization and low conduction velocity of neural fibers, including those of the laryngeal, vagi, and carotid sinus nerves, limit the afferent sensory information involved in the regulation of breathing. Finally, with respect to the adaptation to air breathing at birth, a multitude of events among the most dramatic of the whole life occur quite rapidly, such as the transition from filtration to absorption of the pulmonary fluid, the drastic changes in pulmonary circulation, and the rise in oxygenation with its implications on the function of the chemoreceptors.
), achieved mostly by breathing at high rates. Indeed, fast breathing is a common characteristic of all newborn mammals. With respect to maturation, the development of the lung and respiratory apparatus is never completed at birth, even in the most precocial species. The lungs continue to grow postnatally, in a centripetal direction, with formation of peripheral airways and alveoli, implying that in newborns the central airways comprise a larger proportion of the total air space and contribute to a relatively large anatomical dead space. The incomplete myelinization and low conduction velocity of neural fibers, including those of the laryngeal, vagi, and carotid sinus nerves, limit the afferent sensory information involved in the regulation of breathing. Finally, with respect to the adaptation to air breathing at birth, a multitude of events among the most dramatic of the whole life occur quite rapidly, such as the transition from filtration to absorption of the pulmonary fluid, the drastic changes in pulmonary circulation, and the rise in oxygenation with its implications on the function of the chemoreceptors.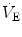 responses by integrating peripheral information with metabolic requirements. The general principles of operation of the neuromechanical unit (that is, translation of neural output into mechanical events, evaluation of the adequacy of
responses by integrating peripheral information with metabolic requirements. The general principles of operation of the neuromechanical unit (that is, translation of neural output into mechanical events, evaluation of the adequacy of 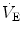 from neurochemical feedback) are the same throughout the whole postnatal life. However, some functional aspects are peculiar to the neonatal period. The goal of this chapter is to highlight the functional properties characteristics of the newborn. References are not extensive, with preference given to review articles where the interested reader can trace the background information and technical details. A short list of terminology and abbreviations adopted in the text is given in Table 2.1.
from neurochemical feedback) are the same throughout the whole postnatal life. However, some functional aspects are peculiar to the neonatal period. The goal of this chapter is to highlight the functional properties characteristics of the newborn. References are not extensive, with preference given to review articles where the interested reader can trace the background information and technical details. A short list of terminology and abbreviations adopted in the text is given in Table 2.1.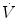
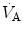
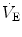
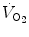
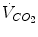
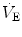 at hatching are believed to be caused by the rise of metabolically produced CO2 (Mortola 2009). Hence, it is quite possible that the risen level in metabolic rate at birth represents the main mechanism sustaining continuous
at hatching are believed to be caused by the rise of metabolically produced CO2 (Mortola 2009). Hence, it is quite possible that the risen level in metabolic rate at birth represents the main mechanism sustaining continuous 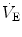 .
.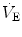 generated by the contraction of the respiratory muscle results from three key steps (Fig. 2.1). First, the intrinsic mechanical characteristics of the muscle fibers determine the force that the muscle is able to generate. Then, the force translates into pressure, the magnitude of which depends on the geometrical characteristics of the muscle and the structure over which the force is applied. Finally, the pressure generates airflow and causes changes in lung volume according to the flow-resistive and elastic properties of the respiratory system.
generated by the contraction of the respiratory muscle results from three key steps (Fig. 2.1). First, the intrinsic mechanical characteristics of the muscle fibers determine the force that the muscle is able to generate. Then, the force translates into pressure, the magnitude of which depends on the geometrical characteristics of the muscle and the structure over which the force is applied. Finally, the pressure generates airflow and causes changes in lung volume according to the flow-resistive and elastic properties of the respiratory system.