The Fetus as a Surgical Patient
Like the prenatal life it purports to treat, fetal surgery as a discipline remains sequestered. Only a handful of centers have developed the complex program required to operate upon fetuses. Despite this, however, fetal surgery is poised on the verge of the surgical mainstream. As patients become more educated about the possibility of fetal surgery, obstetricians and perinatologists face the dilemma of whether or not to explain the option of fetal surgery to parents and then refer the parents for surgical evaluation. The acceptability of fetal surgery is related mainly to improved efficacy and patient selection. Improved efficacy has been achieved in part though incremental advances in techniques and monitoring as well as ingenious applications of unique fetal biology. Improvements in diagnostic technology, coupled with prospective longitudinal evaluation of affected fetuses, have indicated which fetal disorders and, more importantly, which individual fetuses, are best candidates for surgery. Unquestionably, the fetus is now considered to be a patient (Harrison and Adzick, 1991). While maternal safety has been the principal concern, no significant morbidity or mortality has been, fortunately, reported for these mothers (Farmer, 1998; Farrell and colleagues, 1999; Harrison, 1996). The future challenge for fetal surgery is to broaden and refine its applications while incurring minimal maternal risks.
HISTORY
Fetal intervention began with attempts to treat prenatally diagnosed medical conditions. Initial attempts were directed toward the treatment of hemolytic anemia from anti-D isoimmunization. Sir Williams Liley (1963) reported transuterine fetal intraperitoneal red blood cell transfusion, which earned him consideration as the “Father of Fetal Surgery.”
Asensio and colleagues (1966) performed the first successful open fetal surgery for anti-D isoimmunization. As successes accrued, some began to treat sonographically diagnosed fetal fluid collections by catheter decompression. Since then, an extensive experience has accumulated using these procedures for hydrothorax and obstructive uropathy. Thoracoamnionic shunts were used to drain pleural fluid into the amnionic sac. Percutaneously placed catheters to decompress the urinary tract in obstructive uropathy have been effective, but are problematic because of frequent catheter dislodgment and obstruction.
Most fetal malformations are complex and cannot be treated this simply and an open surgical approach must be used. Extensive investigation of open fetal surgery has been undertaken in animal models. The domestic sheep is an ideal model because the fetal lamb has a long gestation period and is highly resistant to preterm labor, thus allowing multiple survival surgeries. Its large size eases the technical demands of fetal manipulation. Major drawbacks are high costs and its many dissimilarities to human physiology and anatomy, particularly regarding the fetal-maternal interface and its quiescent uterus. The most rigorous model is the nonhuman primate, which approximates the human because of its long gestational period, irritable uterus, and anatomic and physiologic similarities.
In this chapter, we describe the preoperative, intraoperative, and postoperative care involved in fetal surgery. Emphasis is placed on the experimental and clinical surgical experiences of leading centers and their relevance to state-of-the art treatment.
GENERAL ASPECTS OF FETAL SURGERY
ULTRASONOGRAPHY
Fetal surgical intervention would not be possible without significant improvements in ultrasonography. Progress with imaging has not only made early prenatal diagnosis of structural anomalies possible, but it has defined their natural history (Shaaban and colleagues, 1999). This is of paramount importance in selecting patients for intervention. It also allows evaluation to exclude those fetuses with complex disorders that will not benefit from treatment. For example, those with complex central nervous system or cardiac disorders cannot be helped with surgery at present. Sonography is also indispensable intraoperatively. It is used to locate and avoid the placenta during open and endoscopic fetal procedures, locating important fetal anatomic landmarks, and aiding in the determination of fetal well-being.
JUSTIFICATION FOR FETAL INTERVENTION
Guidelines for selection criteria for prenatal surgery, shown in Table 36-1, are regularly updated by the International Fetal Medicine and Surgery Society. Fetal surgery is ideally considered after all of these criteria are met. Importantly, the natural history of the disorder must also be known, prenatal diagnosis must be accurate, and multiple anomalies must be excluded. Perhaps most importantly, operative procedures should only be performed at institutions with an appropriately trained multidisciplinary team.
TABLE 36-1. Criteria for Fetal Surgery
TECHNIQUES OF OPEN FETAL SURGERY
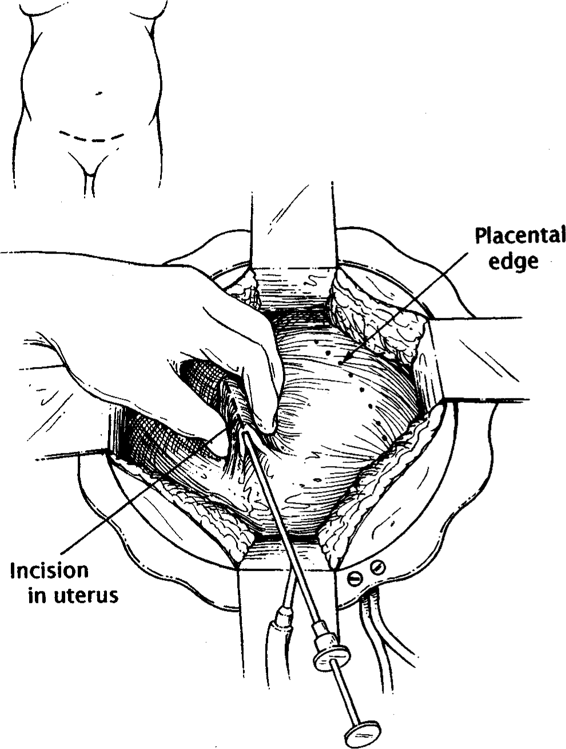
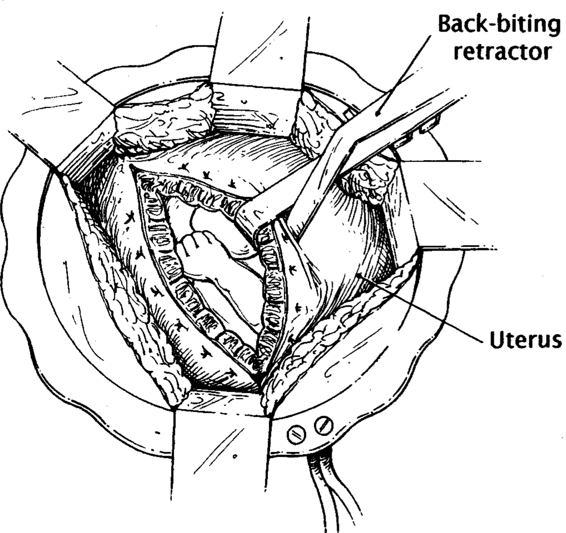
Fetal surgical intervention has provided a host of new technical obstacles. Techniques devised to open and close the uterus are fundamental to successful open surgery. The technique of Harrison and Adzick (1991) is briefly described: A Pfannenstiel incision is used to deliver the uterus extra-abdominally. A ring retractor with a built-in circular antenna is used to simultaneously retract the abdominal wall and improve reception of the intraoperative fetal electrocardiography (ECG) signal from a customized radiotelemetry device. Intraoperative sonography is used to identify and mark placental location. After the uterus is completely relaxed, a biliary trocar is placed into the uterus, amnionic fluid is aspirated, and the uterus is compressed with the fingers onto the trocar while cautery is used on the trocar to open a small hysterotomy (Fig. 36-1). Next, because of the vascular nature of the uterus, a specially designed absorbable stapler device (U.S. Surgical, Norwalk, CT) is used to cut and staple the uterus simultaneously (Fig. 36-2). The fetus is continuously irrigated with warm saline to maintain body temperature and prevent skin desiccation.
FIGURE 36-1. The maternal abdomen is opened, exposing the uterus. Hysterotomy is performed over a biliary trocar after the placental edge is localized using ultrasonography. (Redrawn from Hedrick and colleagues, 1998, with permission.)
FIGURE 36-2. The uterus is opened with a specialized stapler that has absorbable sutures, and retracted to expose the fetus. (Redrawn from Hedrick and colleagues, 1998, with permission.)
After surgery is complete and the umbilical cord positioning is optimized, the fetus is returned to the uterus. Amnionic fluid is reconstituted with isotonic electrolyte solution at physiologic temperature. The uterus is closed in three layers and returned to the abdominal cavity. The incidence of maternal complications from hysterotomy and fetal exposure has been low. Minimally invasive techniques of fetal intervention may help solve some of the more vexing problems of fetal surgery such as hysterotomy-induced preterm labor and fetal demise.
TECHNIQUES OF MINIMALLY INVASIVE FETAL INTERVENTION
Fetoscopy represents a promising technique for fetal intervention. Advances in camera/video quality, fiberoptic lighting, and surgical instrumentation have dramatically improved the ability to perform complex fetal manipulations. The most important benefit is the avoidance of a hysterotomy, and thus a reduction in preterm labor. Uterine activity is reportedly absent in Rhesus monkeys after fetal endoscopic surgery (van der Wildt and associates, 1995).
There are several reports of human fetoscopic intervention. In twin pregnancies with twin-twin perfusion abnormalities, Quintero and colleagues (1995) reported fetoscopic umbilical cord ligation. Similarly, De Lia and coworkers (1995) reported laser ablation of placental vessels to successfully improve the status of the nonparasitic twin. Even though the present techniques of fetoscopic intervention are crude relative to the current standards of minimally invasive surgery in other disciplines, progress in overcoming the technical limitations is rapid.
Many details must be understood before safe application of fetoscopic techniques can be clinically applied. For example, the proper substance for insufflation and appropriate pressure levels must be determined. Techniques must be established to access the fetus and perform complex manipulations while maintaining uterine integrity. Carbon dioxide uterine insufflation may be deleterious because of fetal acidosis and hypercarbia. Pelletier and colleagues (1995) showed that helium may be preferable to CO2 and that an insufflation pressure of 15 mm Hg is well tolerated by the fetus. Skarsgard and associates (1995) used warm saline infusion and demonstrated that an amnionic fluid pressure of 20 mm Hg or less preserves placental perfusion. Current fetoscopic techniques utilize high-volume infusion to rapidly replace amnionic fluid. This simultaneously maintains good visibility and a “fetal-friendly” environment.
MATERNAL-FETAL PERIOPERATIVE CARE: THE FETAL INTENSIVE CARE UNIT
Care of both mother and fetus requires a multidisciplinary approach that transcends the expertise of individual specialties. Maternal-fetal medicine physician and nurses, pediatric surgeons, neonatologists, intensivists, and geneticists are among the many that comprise the fetal treatment team that makes complex decisions. Maternal safety is the principal concern in open fetal surgery.
In the early 1980s, fetal primate surgery was used to develop the anesthetic, surgical, and tocolytic regimens for human fetal surgery (Adzick and colleagues, 1986; Harrison and associates, 1982). In 1991, Longaker and coworkers reviewed the first 17 fetal surgery cases and concluded that hysterotomy can be accomplished without maternal danger. Farrell and coworkers (1999) recently reported that open fetal surgery had a negligible impact on maternal fertility. Maternal monitoring is accomplished with the standard techniques including pulse oximetry and an arterial line. Frequently, a pulmonary arterial line is placed to monitor left ventricular filing pressures and cardiac contractility to maximize maternal-fetal hemodynamics.
Intraoperatively, fentanyl, pancuronium, and halogenated anesthetics are given which provide anesthesia for both patients (Harrison and colleagues, 1982). Until recently, intraoperative and postoperative fetal monitoring was inadequate. Many fetal operations that are now performed, if they were performed on a neonate, would require intensive care monitoring. Currently, there are no mechanisms to treat postoperative fetal complications. Perioperative fetal monitoring techniques continue to evolve by trial and error. A transcutaneous pulse oximeter probe is placed around the fetal thigh or arm for continuous measurement of oxygen saturation and heart rate. Luks and associates (1998) recently concluded that fetal pulse oximetry in the lamb had a rapid response time, high sensitivity, and a 100 percent negative predictive value. Other parameters, including blood pressure and bradycardia, were found to be either too invasive or too insensitive.
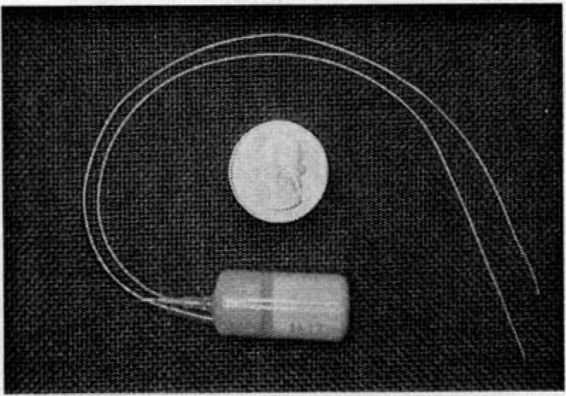
A specifically developed implantable radiotelemeter (Fig. 36-3), which is placed on the fetus at the beginning of the open procedure, allows continuous recordings of the fetal electrocardiogram and intrauterine pressure during and after surgery (Jennings and coworkers, 1993). This device has dramatically improved the ability to monitor both fetal well-being and uterine activity. The two electrocardiographic leads and the housing of the device are sutured to the skin intraoperatively and the pressure catheter is allowed to float freely within the amnionic sac. Intraoperative ultrasound is used throughout the operation to aid in assessment of fetal heart rate, volume status, and cardiac contractility. Umbilical cord blood can be sampled, if necessary, to evaluate fetal electrolytes, hemoglobin, or pH. Should the fetus require intravenous therapy, a small butterfly catheter can be introduced into the umbilical cord for the administration of packed red cells, pressors, and fluid and electrolytes.
FIGURE 36-3. An implantable fetal radiotelemeter monitor (Data Sciences International, St. Paul, MN) transmits fetal electrocardiographic and intrauterine pressure information to an external receiver. (Reprinted from Hedrick and coworkers, 1998, with permission.)
At the conclusion of the procedure, fetal monitoring becomes significantly more difficult with the loss of direct physical access. True fetal intensive-care monitoring is the ultimate goal for postoperative management. While maternal well-being was often thought to imply fetal well-being, this is not the case. Despite optimal maternal care, inexplicable fetal demise remains a troubling problem. Sonography is used for intermittent study of fetal well-being and amnionic fluid volume. Echocardiography is used to evaluate fetal hemodynamic function. The implantable radiotelemeter continuously records intra-amnionic fluid pressure as well as the electrocardiographic tracing. Patterns are used to herald the onset of labor, predictable in the posthysterotomy uterus, and then to alter the maternal tocolytic regime.
Brain injury after fetal surgery has been studied. In one study, 7 of 33 fetuses had significant injuries consisting of ventricular or periventricular hemorrhage and periventricular leukomalacia (Bealer and colleagues, 1995). Intensive postnatal care, intracranial hemorrhage, and prolonged respiratory support were associated with a worse neurologic prognosis (Gibbs and coworkers, 1998). Ultrasonography is currently being used to serially evaluate these fetuses.
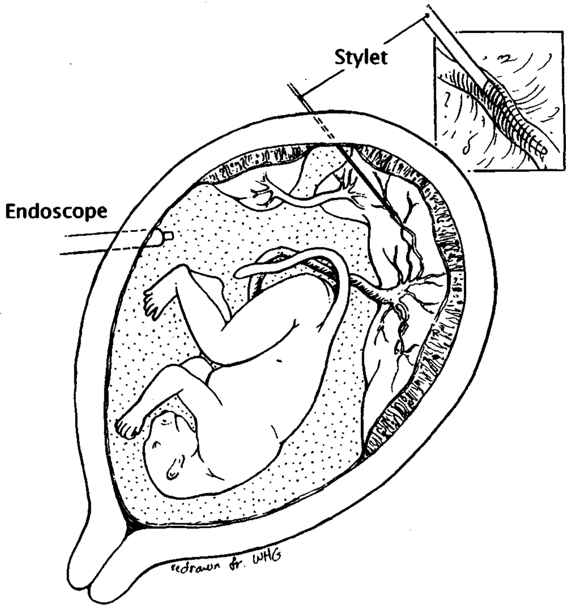
True fetal intensive care will require chronic postoperative access to the fetal circulation. Fetoscopic extra-amnionic catheterization of placental vessels has been used successfully and safely in primates to transduce fetal blood pressures, sample fetal blood, and infuse medications (Fig. 36-4). This technique may soon be applicable in human fetal surgery and may be useful for the more general problem of continuous delivery of substrates or specific cell lines to correct a variety of fetal diseases (Hedrick and coworkers, 1993). In the future, it may be possible to care for the fetus independently; such extrauterine techniques have been studied in animal models.
FIGURE 36-4. Technique of placental vessel catheterization. (Redrawn from Hedrick and associates, 1993, with permission.)
Attempts have been made to develop an “artificial placenta” (Sakata and colleagues, 1998; Zapol and associates, 1969). These attempts include long-term extrauterine support of goat fetuses ranging from 10 days to 3 weeks. Arteriovenous extracorporeal membrane oxygenation (ECMO) via the umbilical vessels was used, and goat fetuses were incubated in lactated Ringer solution. With the rapid improvements in ECMO over the past few years, artificial placentas will undoubtedly continue to improve.
FETAL SURGERY AND PRETERM LABOR
Postoperative tocolysis is a frustrating problem. Marked and predictable uterine irritability occurs after open hysterotomy. In many cases, a successful fetal operation was nullified because of uncontrollable preterm labor. Treatment with β-agonists, magnesium sulfate, indomethacin, and other agents, although routinely used, has been incompletely effective. Nitric oxide (NO) donors inhibit preterm labor after open fetal surgery in monkeys (Jennings and collaborators, 1993). Nitroglycerine is a potent NO donor that may have beneficial vasodilatory effects on uterine and placental blood flow. Nitroglycerine infusion has been used postoperatively; unfortunately, significant doses result in maternal hypotension, which is treated aggressively with intravenous fluids. Women are kept at strict bedrest in the immediate postoperative period.
Nitroglycerine use is associated with acute pulmonary edema at a much higher rate than that seen among obstetric patients (DiFrederico and coworkers, 1998). Pulmonary disease is more severe and has longer resolution times than with other tocolytic agents. A recent randomized study of preterm labor showed that nitroglycerine was not as efficacious as magnesium sulfate to prevent preterm labor (El-Sayed and colleagues, 1999). In addition, a fourth of the nitroglycerine group had persistent hypotension requiring discontinuation of the therapy.
SPECIFIC FETAL DISORDERS
NECK ABNORMALITIES
CONGENITAL HIGH AIRWAY OBSTRUCTION SYNDROME. Congenital obstruction of the upper airway is easily recognized by ultrasonography, and a number of cases have been reported (Richards, 1992; Watson, 1990; Weston, 1992, and their colleagues). Hedrick and associates (1994) termed the condition as congenital high airway obstruction syndrome (CHAOS). All fetuses with CHAOS exhibit similar sonographic findings including large echogenic lungs, flattened or inverted diaphragms, dilated airways distal to the obstruction, and fetal ascites and/or hydrops (Fig. 36-5). Although appropriate perinatal management of the occluded airway can be lifesaving, no fetus has survived.
FIGURE 36-5. Drawing of a fetus with congenital high airway obstruction syndrome (CHAOS), showing clinical and sonographic findings. (Redrawn from Hedrick and coworkers, 1994, with permission.)
Laryngeal atresia is the most common cause of CHAOS (Hedrick and associates, 1998). Experimental tracheal ligation has shown that the airway obstruction must be early and relatively complete to be symptomatic. Tracheal atresia does not produce CHAOS because of its association with a tracheoesophageal fistula, which acts as a conduit to vent intra-bronchial accumulation of excess lung fluid.
Ultrasonic findings of CHAOS are manifestations of an obstructed upper airway. The fetal lungs are net fluid producers (Carmel and colleagues, 1965). Normally, fluid flows through the fetal airway and becomes a component of the amnionic fluid. Obstruction results in lung expansion, which inverts the diaphragm and dilates the airways. Ascites and nonimmune hydrops are secondary responses, presumably due to direct cardiac and caval compression by the massively distended lungs. Hydramnios, possibly due to esophageal compression and impaired fetal swallowing, may be associated with CHAOS and predispose to preterm delivery, placentomegaly, and preeclampsia (Watson and coworkers, 1990). This last constellation of findings is an example of the maternal “mirror syndrome,” in which a hydropic fetus induces a preeclamptic-like condition (Adzick and colleagues, 1993).
Management of the fetus with CHAOS is unclear, although several principles seem reasonable. A thorough fetal sonographic examination is done to rule out other significant anomalies. The ex utero intrapartum treatment (EXIT) procedure, also called operation on placental support (OOPS), has been extrapolated to fetuses with CHAOS and large neck tumors (DeCou and colleagues, 1998; Skarsgard and associates, 1996). Fetuses remain on placental support, before the cord is divided, for as long as 60 minutes (Jona, 1998). During this time, the fetus can undergo multiple procedures, including tracheostomy, securing the airway from extrinsic obstruction from hemangiomas or lymphatic malformations, or removal of the tracheal clip placed at congenital diaphragmatic hernia repair. A recent case report described a successful EXIT procedure done on a 33-week fetus for a large anterior neck teratoma (Shih and coworkers, 1998.)
VASCULAR MALFORMATIONS. Arteriovenous malformations (AVM), hemangiomas, and lymphatic malformations may occur anywhere, but are concentrated in the neck region. They can be diagnosed by sonography, and in general, these lesions rarely cause significant fetal problems. Giant cavernous hemangiomas may produce significant increases in cardiac output, nonimmune hydrops, and fetal demise. For most fetuses with large vascular lesions of the head and neck, perinatal management consists of careful attention to airway management. This may necessitate cesarean delivery and the EXIT procedure as described above.
THORACIC DISORDERS
CONGENITAL DIAPHRAGMATIC HERNIA. The incidence of these hernias is 1:2500 to 1:5000 births (Shaaban and colleagues, 1999). The hernia is most often a posterior-lateral defect in the left hemidiaphragm. Neonates born with severe herniation die of pulmonary insufficiency because the herniated viscera prevents normal lung growth. Before fetal surgical intervention for congenital diaphragmatic hernia (CDH) could be attempted, a thorough understanding of the prenatal and postnatal natural history was necessary. A significant amount of experimental work in lambs was performed to prove the feasibility of human fetal intervention (Harrison, 1991).
Survival rates with CDH depend on when the defect is diagnosed. Tertiary referral centers with ECMO report survival rates of about 75 percent (Atkinson and colleagues, 1991; Van Meurs and associates, 1999). Alternatively, if all fetuses diagnosed prenatally with CDH are considered, the survival rate is much lower. In a prospective study of 52 fetuses diagnosed prenatally, only 40 percent survived despite optimal postnatal care (Harrison and colleagues, 1993). Of these 52 fetuses, 10 died in utero or in transit to a referral center. These deaths represent the “hidden mortality” of CDH in most studies.
Understanding the concept of hidden mortality associated with fetal diseases is crucial to understanding the rationale of fetal intervention. Because many fetuses with CDH do well, criteria are needed to properly select those with the poorest chance of survival. Diagnosis prior to 25 weeks, lung-to-head ratio (LHR), dilated stomach, liver incarcerated into the chest, and associated congenital anomalies have been evaluated. In a review, Metkus and colleagues (1996) found that the only significant predictor of survival was lung-to-head ratio. In a prospective study, Lipshutz and coworkers (1997) reported that all fetuses with an LHR <1.0 died, and all with an LHR >1.4 survived. The LHR is not a replacement for other sonographic predictors of outcome, including gestational age and liver incarceration, but it is a supplemental predictor. Magnetic resonance imaging (MRI) may be better than sonography in determining location of the fetal liver, which has extremely important therapeutic ramifications (Hubbard and associates, 1997).
The decision to perform CDH repair prenatally was evaluated prospectively by Harrison and colleagues (1997) at the UCSF Fetal Treatment Center. Four fetuses without liver herniation underwent open surgery for repair, while seven were treated conventionally. There was no difference in survival, length of ventilatory support, and requirement for ECMO of neonates in each group. They concluded that although fetal surgery for CDH is physiologically sound and technically feasible, it does not currently afford better outcomes.
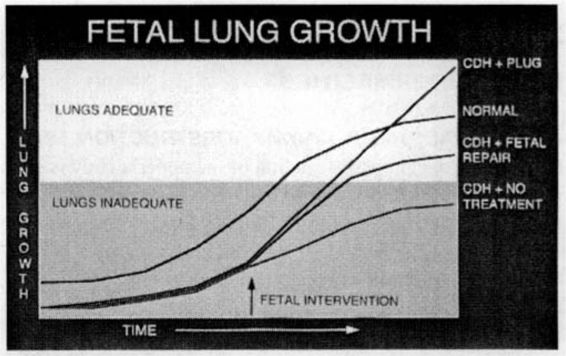
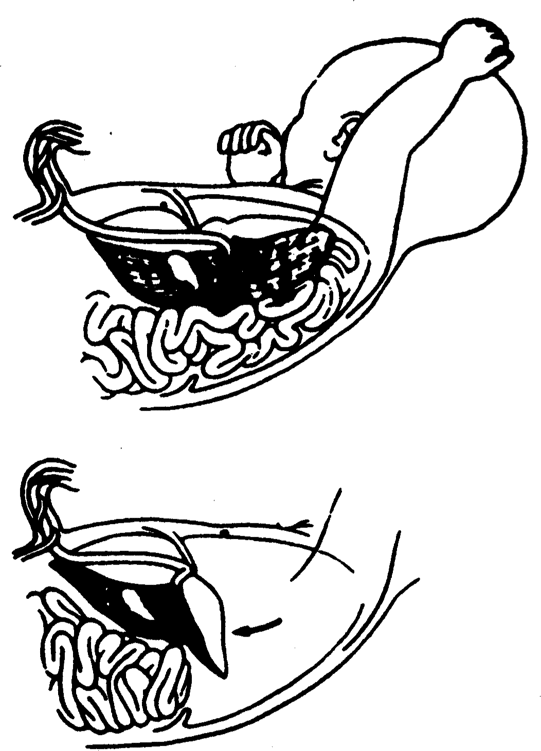
In 1994, Hedrick and associates reported the PLUG technique (plug the lung until it grows) to treat fetuses with CDH. This technique takes advantage of the clinical observation that fetuses with congenitally atretic or obstructed airways are born with massively enlarged lungs or CHAOS. When obstruction is applied experimentally to fetuses with CDH, lung growth and function is substantially improved (Fig. 36-6). The UCSF group evaluated the possibility of open fetal surgery with tracheal occlusion for CDH with liver herniation (Fig. 36-7). Temporary occlusion of the fetal trachea accelerates fetal lung growth and resolves pulmonary hypoplasia associated with severe CDH. Unfortunately, complications from the open technique have limited the survival rate.
FIGURE 36-6. Fetal lung growth after various forms of prenatal treatment for congenital diaphragmatic hernia. Normal fetal lungs are used as reference. (Redrawn from Hedrick and colleagues, 1998, with permission.)
FIGURE 36-7. Acute reduction of the liver from the left chest causes occlusion of the umbilical vein, and subsequent termination of blood return from the placenta. (Redrawn from Harrison, 1991, with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree