Management of Postpartum Hemorrhage
Despite widespread recognition of the consequences of obstetric hemorrhage and the availability of modern blood-banking techniques, postpartum bleeding remains a major source of maternal morbidity and mortality in the United States and in developing countries. Recent surveys suggest that postpartum hemorrhage accounts for about 10 percent of maternal deaths in the United States (Rochat and colleagues, 1988). If ectopic pregnancy is included, hemorrhage is the leading cause of maternal mortality in this country. In developing countries, obstetric hemorrhage is an even more important cause of maternal death (Harrison, 1989; Rosenfield, 1989).
There is no universally accepted definition of postpartum hemorrhage. Traditionally, blood loss exceeding 500 mL following vaginal delivery has been defined as postpartum hemorrhage. In practice, clinical estimate of blood loss is oftentimes inaccurate and usually significantly less than this value. It has been estimated that blood loss is frequently underestimated as much as 30-50 percent (ACOG, 1998). However, several studies using quantitative techniques of blood loss assessment suggest a mean blood loss following uncomplicated vaginal delivery of about 500 mL. This compares with a 1000 mL blood loss for cesarean delivery, 1400 mL for elective cesarean hysterectomy, and 3000-3500 mL for emergency cesarean hysterectomy (Table 23-1). In a review of 19 studies of postpartum blood loss, Gahres and associates (1962) reported a mean loss following vaginal delivery of 450 mL for all series. In view of such data, it is clearly inappropriate to perpetuate the traditional definition. The American College of Obstetricians and Gynecologists (ACOG, 1989) has suggested that postpartum hemorrhage be defined as a fall in hematocrit peripartum of at least 10 percent or hemorrhage requiring blood transfusion. Gilstrap and Ramin (1994) suggested that postpartum hemorrhage is that amount of bleeding resulting in hemodynamic instability or that will result in such if left unabated. The precise amount of bleeding that meets this latter criterion varies depending on several factors, paramount of which is the degree of circulating blood volume in a given woman. For example, a blood loss of 1000 mL may be of little consequence following an otherwise uncomplicated twin delivery, while such a loss might cause significant hypotension in a woman with severe preeclampsia. Chua and colleagues (1998) recently described a laboratory method involving extraction and the photometric measurement of alkaline hematin to provide an accurate measurement of postpartum blood loss.
TABLE 23-1. Estimated Mean Blood Loss for Obstetric Procedures
Significant postpartum hemorrhage is estimated to complicate 2-4 percent of vaginal deliveries and 6 percent of cesarean births (Combs and colleagues, 1991a,b; Mee, 1990). In women delivering vaginally, risk factors for postpartum hemorrhage include prolonged third-stage labor; pre-eclampsia; episiotomy; previous postpartum hemorrhage; twins; arrest of descent; soft-tissue lacerations; oxytocin-augmented labor; instrumental vaginal deliveries; nulliparity; Asian or Hispanic ethnicity; and obesity (Combs and associates, 1991a). Risk factors for hemorrhage following cesarean delivery include classical uterine incision; am-nionitis; preeclampsia; abnormal labor; general anesthesia; pre- and postterm delivery; and Hispanic ethnicity (Combs and coworkers, 1991b; Gilstrap and associates, 1987).
An intrinsic lack of constrictor reactivity in the uterine arteries of women with various types of postpartum hemorrhage has also been described (Nelson and Suresh, 1992). It is difficult to separate this deficiency from secondary local effects of hypoxia/ischemia in bleeding hypotensive patients.
HYPOVOLEMIC SHOCK
In general, the hypervolemia with accompanying expanded red cell mass of pregnancy allows the parturient to accommodate normal to even excessive blood loss at vaginal delivery without a decrease in postpartum hematocrit. In one study, the mean postpartum hematocrit decrease was 2.6 ± 4.3 percent and one-third of women either had no decline or had an actual increase (Combs and associates, 1991a). Women undergoing cesarean section experienced a mean drop in hematocrit of 4.0 ± 4.2 percent after equilibration, but about 20 percent had no decline (Combs and colleagues, 1991). Even with moderate additional blood loss, venous compensatory mechanisms allow most women to tolerate such hemorrhage without hemodynamic compromise. With continued hemorrhage, however, these mechanisms may be overwhelmed with resultant hypotension, decreased tissue perfusion, cellular hypoxia, and death. In fact hemorrhage is the most common cause of shock in obstetrics and gynecology(ACOG, 1997).
Hypovolemic shock evolves through severe stages. Early in the course of massive hemorrhage, there are decreases in mean arterial pressure, cardiac output, central venous pressure, and stroke volume. Increases in arterial-venous oxygen content difference reflect a relative increase in tissue oxygen extraction, although overall oxygen consumption falls (Bland and colleagues, 1985; Shoemaker, 1973; Shoemaker and associates, 1979a,b).
At least 70 percent of total blood volume is contained in venules, which are passive resistance vessels controlled by humoral factors. Catecholamine release during hemorrhage causes a generalized increase in venular tone, resulting in an autotransfusion from this capacitance reservoir. These changes are accompanied by compensatory increases in heart rate, systemic and pulmonary vascular resistance, and myocardial contractility. In addition, there is redistribution of cardiac output and blood volume by selective central nervous system-mediated arteriolar constriction. This results in diminished perfusion to the kidneys, splanchnic beds, skin, and uterus with relative maintenance of blood flow to the heart, brain, and adrenal glands (Shoemaker and colleagues, 1967; Slater and associates, 1973).
As blood volume deficit exceeds a certain critical level, such compensatory mechanisms usually are inadequate to maintain cardiac output and blood pressure. At this point, additional small losses of blood result in rapid clinical deterioration. Despite an initial increase in total oxygen extraction by maternal tissue, maldistribution of blood flow results in local tissue hypoxia and metabolic acidosis, producing a vicious cycle of vasoconstriction, organ ischemia, and cellular death, leading to loss of capillary membrane integrity and additional loss of intravascular volume (Slater and coworkers, 1973). Increased platelet aggregation is also found in hypovolemic shock, resulting in the release of a number of vasoactive mediators, which cause small vessel occlusion and further impairment of microcirculatory perfusion.
MANAGEMENT
Proper management of postpartum bleeding requires a thorough search for the specific cause of the hemorrhage. Individual diagnoses in the differential diagnosis require specific approaches to management, both in terms of technique and in the rapidity with which such techniques are applied. Proper management of intermittent uterine atony, for example, may at times involve a prolonged period of uterine massage, observation, and continued attempts at pharmacologic reversal. On the other hand, postpartum hemorrhage from placenta accreta will generally require prompt hysterectomy. Errors can be made when the clinician manages “postpartum hemorrhage” without attempts to determine specific etiology.
Fortunately, the causes of severe postpartum hemorrhage are few and, in virtually all cases, involve one of four factors: uterine atony, retained placenta (including placenta accreta and other abnormal attachment), upper or lower genital tract lacerations, and coagulopathy. For the woman experiencing hemorrhage at the time of cesarean, the cause is often immediately apparent. Table 23-2 presents a management scheme for the evaluation of hemorrhage following vaginal delivery.
TABLE 23-2. Evaluation of Postpartum Hemorrhage
1. Palpate the uterus to rule out atony.
2. Inspect the lower genital tract for lacerations.
3. Examine the placenta and uterine cavity for retained placenta.
4. Consider causes of coagulopathy.
5. Perform exploratory laparotomy.
ATONY
Uterine atony is the most frequent cause of serious postpartum hemorrhage. Risk factors for uterine atony include advanced parity, the use of oxytocin for labor augmentation, magnesium sulfate infusion, chorioamnionitis, arrest disorders of labor, and uterine overdistension, as in macrosomia or multiple gestation. In the series described by Clark and colleagues (1984), 80 percent of women undergoing hysterectomy for intractable atony had one or more of these risk factors. Recognition of such factors may allow the clinician to anticipate this problem. Bleeding from atony may be rapid and leave little time for indecision, and there must be a well-established management protocol, such as that shown in Table 23-3, designed to reverse atony.
TABLE 23-3. Management of Uterine Atony
2. Oxytocin 40 U/L–rapid infusion
3. 15-methyl PGF2α0.25–1.5 mg intramuscular or intramyometriala
4. Uterine artery ligation
5. Multiple uterine sutures
6. Internal iliac artery ligationb
7. Hysterectomy
aAlternatives include PGE2α, 20 mg suppository (rectally), methylergonovine 0.2 mg intramuscularly, and other approaches described in text.
bPrerequisites: hemodynamic stability, desirous of future childbearing, operator experience.
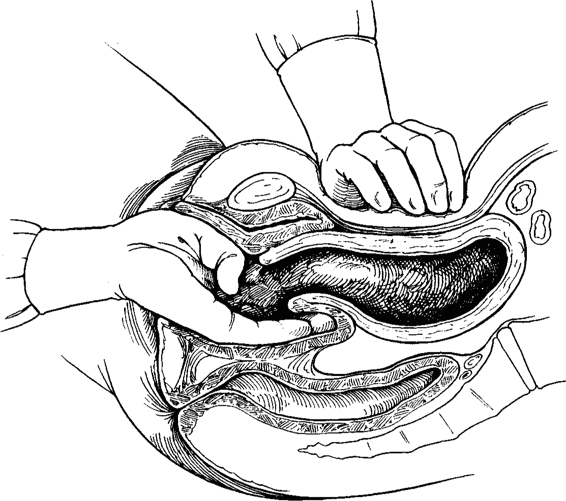
If atony is encountered following removal of the placenta, firm compression is exerted on the uterus. This may be performed transabdominally; however, in many cases, bimanual compression is more effective (Fig. 23-1). Even if atony does not promptly resolve, such compression can often provide temporary effective control of hemorrhage while additional pharmacologic manipulation is attempted, blood products are obtained, and assistance is summoned. Simultaneous with such compression, a dilute solution of 40 units of oxytocin in 1000 mL of crystalloid is infused rapidly. Because rapid crystalloid infusion is the first step in management of such women, the intravenous line can be opened fully. The direct intravenous bolus injection of oxytocin may cause a paradoxical hypotensive response, which could be detrimental to an already hypovolemic patient and is, therefore, contraindicated (Secher and colleagues, 1978).
FIGURE 23-1. Bimanual compression. The vaginal hand should also lift the uterus out of the pelvis and towards the abdominal hand.
If these initial attempts to reverse uterine atony are unsuccessful, additional pharmacologic maneuvers are indicated. A number of studies have demonstrated the effectiveness of various prostaglandin preparations in reversing uterine atony. In the United States, the most commonly used is 15-methyl-PGF2α, 0.25–1.5 mg. It may be administered as an intramuscular injection, or given directly into the myo-metrium, transabdominally, or transvaginally. Each method has proponents; a critical review of the available data, however, does not suggest a clear general superiority of any route of injection (Bittino, 1986; Bruce, 1982; Hayashi, 1984; Toppozada, 1981; and their colleagues).
The use of intravaginal or intrarectal 20 mg PGE2 suppositories has also been described, as has the direct intrauterine instillation of a solution of PGE2 following occlusion of the cervix with a balloon catheter (Hertz and colleagues, 1980; Oleen and Mariano, 1990; Peyser and Kupferminc, 1990). Additional prostaglandin analogues, such as PGE1, have also been used successfully (O’Brien and colleagues, 1998). Rectal misoprostol has also been utilized in an attempt to prevent postpartum hemorrhage (Bamigboye, 1998; Ramsey, 1999; and their colleagues).
The use of uterine packing for atony following failure of pharmacologic therapy is controversial but may provide he-mostasis in some women. Uterine packing may also be useful as a temporary method to decrease bleeding to a degree to allow for adequate blood replacement prior to attempting surgical control of hemorrhage.
In the past, ergot derivatives, principally methylergonovine in doses of 0.2 mg, were commonly given intramuscularly and repeated as necessary. This drug must be used with caution in a previously hypertensive woman. Methylergonovine should not be utilized intravenously. Although the use of methylergonovine remains acceptable, prostaglandin derivatives have relegated such preparations to less frequent use.
At times, atony may be intermittent, requiring repeated physical or pharmacologic intervention over a period of several hours. Under such circumstances, the utmost care must be taken both to assess the amount of blood loss and to assure adequate fluid and blood replacement and hemodynamic stability.
If, following the above maneuvers, the patient with atony continues to bleed significantly, exploratory laparotomy is indicated.
LACERATIONS
Genital-tract lacerations are another frequent cause of post-partum hemorrhage. Lower-tract injuries with bleeding from the vagina, cervix, or ischiorectal fossa are most commonly encountered following vaginal delivery with an unscarred uterus. Uterine rupture with vaginal and/or intraperitoneal bleeding or a retroperitoneal hematoma is uncommon following vaginal delivery in the absence of a prior uterine scar, but occasionally is encountered in multiparous women.
Following vaginal delivery, careful inspection of the vagina and cervix is mandatory if there is bleeding. Lacerations of the vagina, cervix, and perineum are addressed as outlined in Chapter 12. Cervical lacerations are common and, in general, are not repaired unless they are bleeding.
Lacerations encountered at the time of cesarean section most commonly result from lateral or inferior extensions of a lower-segment transverse incision. Because of the proximity of the uterine vessels to the margins of the standard low-transverse incision, such lacerations commonly bleed profusely. In the series by Clark and associates (1984), blood loss associated with hysterectomy for uterine lacerations exceeded that from any type of hemorrhage. Occasionally, such lacerated vessels may temporarily constrict and retract away from the site of primary laceration. Relaxation following abdominal closure may result in delayed intra- or retroperitoneal bleeding, despite meticulous surgical technique.
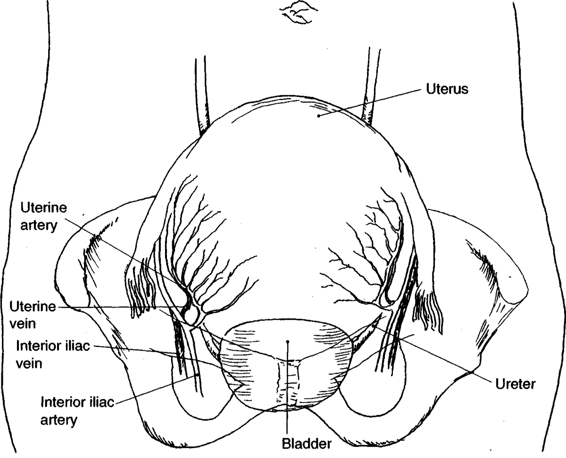
The surgical approach to lateral or inferior lacerations involves awareness of the relative location of uterine vessels, bladder, and ureters (Fig. 23-2). In many cases, the apex of the laceration falls well short of the broad ligament or bladder and repair is straightforward. Hemostasis is achieved with a running locked absorbable suture beginning just beyond the apex of the laceration. When profuse bleeding from the edges of the laceration obscures precise identification of the apex, a running locked absorbable suture placed in one side of the laceration beginning proximally and extending toward the apex may decrease bleeding and facilitate identification of the extent of laceration as the apex is approached (Fig. 23-3).
FIGURE 23-2. Relative postcesarean location of uterine vessels, bladder, and ureters.
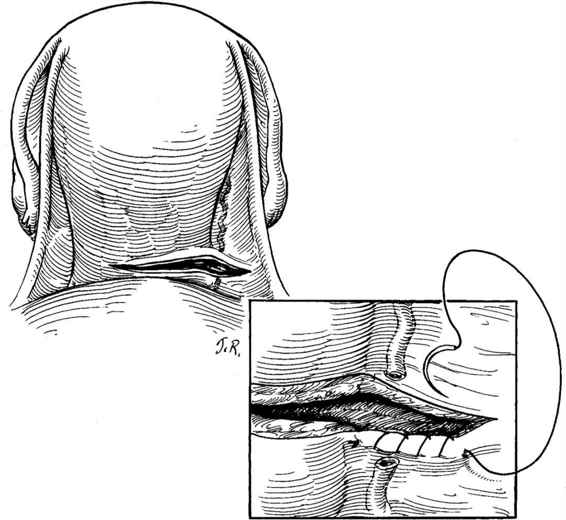
FIGURE 23-3. Identification of apex of lateral uterine laceration when bleeding obscures direct visualization. A running, locked absorbable suture is placed in one margin and run toward the apex, reducing blood loss and facilitating identification of the laceration apex.
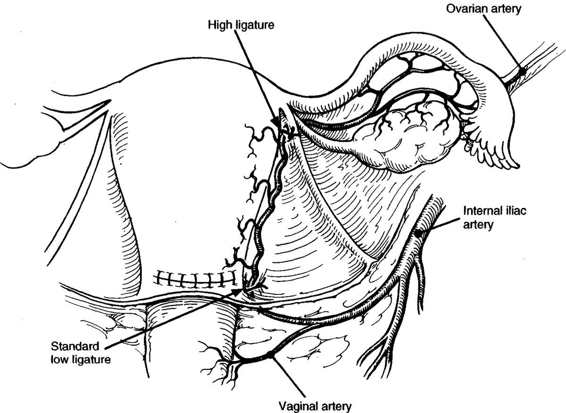
Two situations may be encountered that call for modification of the standard technique of laceration repair; uterine vessel laceration and extension of the incision into the broad ligament. In the first case, when inspection of the lateral laceration reveals profuse bleeding from the lateral margins, involvement of the uterine veins or artery must be suspected. Occasionally, the transected vessel itself is identified. More commonly, bleeding is profuse and palpation of the myometrium-broad ligament junction confirms extension into the area of the uterine vessels. Hemostatic figure-of-eight sutures may be placed in the area, keeping in mind the proximity of the ureter. Often, however, a single, wide suture ligature encompassing the uterine vessels at a point just inferior to the level of the incision may be simpler and result in complete hemostasis. The latter approach is generally preferred to multiple “blind” sutures at the margin of the incision, which may give rise to additional bleeding points and endanger the integrity of the ureter (Fig. 23-4).
FIGURE 23-4. Uterine artery ligation performed at approximate level of the utero-ovarian ligament/uterine junction superiorly and just below the uterine incision inferiorly.
In the second case, the laceration extends into the broad ligament and the relative position of the laceration apex and ureter will not be clear. This situation is uncommon with direct lateral extensions, but is frequently encountered with lateral-inferior extensions. Under such circumstances, it is essential to open the broad ligament and positively identify the course of the ureter prior to suture placement.
IDENTIFYING THE URETER
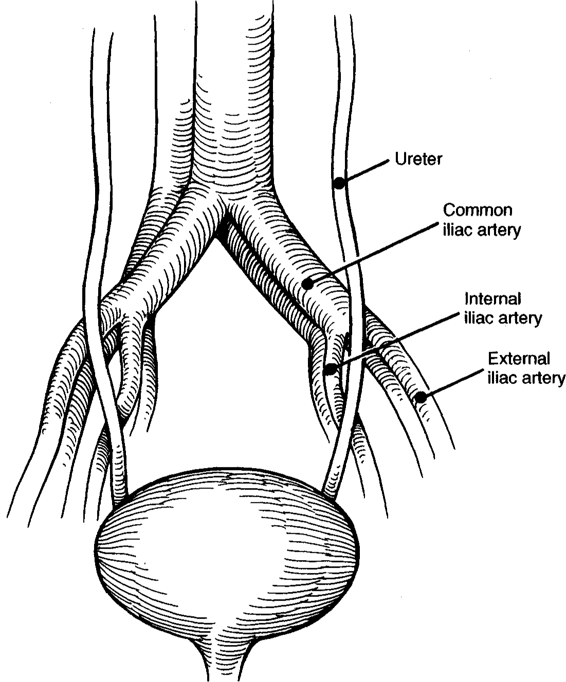
One effective approach is to incise the peritoneum of the broad ligament between the round ligament and fallopian tube. The incision is then extended with Metzenbaum scissors. With experience, the ureter can be readily approached by careful blunt dissection of the areolar tissue in the retroperitoneal space. The ureter is usually easily visualized and can be palpated on the medial leaf of the broad ligament. When difficulty is encountered, the following technique is effective: First, a finger is gently placed into the retroperitoneal space laterally and a pulsating artery identified by palpation. This is usually the external iliac artery. Using a tonsil-tip suction device or a small “peanut” sponge stick, the areolar tissue surrounding the external iliac artery is partially cleared and the artery is followed proximally to the bifurcation of the common iliac artery. Recalling the course of the ureter on the right or left side (Fig. 23-5), it is now easily identified as it crosses the major arteries. Observation of peristalsis, either spontaneous or induced by gentle pressure with an instrument confirms the identity of the ureter. Following identification, the course of the ureter may be traced distally to the area of laceration and appropriate hemostatic sutures can be placed following direct ureteral visualization in this area. This procedure is generally bloodless; however, occasionally small bleeding vessels may be encountered prior to closure of the retroperitoneal space. Use of a lap sponge and pressure or small hemostatic clips is invaluable in obtaining hemostasis under these circumstances and is often preferable to suture placement or extensive cautery. After hemostasis has been achieved, the peritoneum and broad ligament is closed with a running suture of fine absorbable material.
FIGURE 23-5. Course of ureter at bifurcation of common iliac arteries.
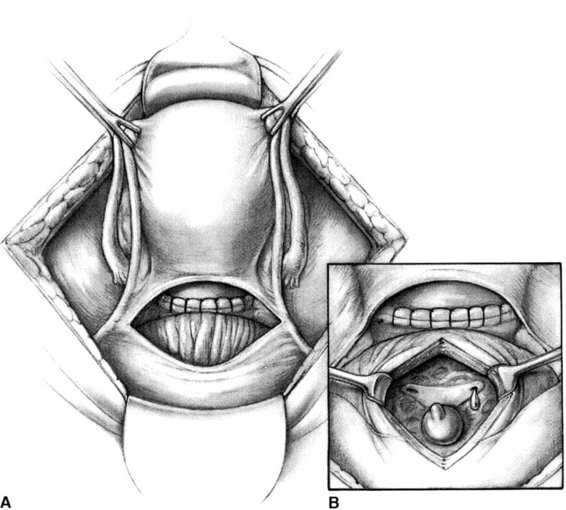
If the integrity of the ureter is in doubt after hemostasis has been achieved, several approaches are possible. The broad ligament may be opened and the course of the ureter directly identified as above. If the tissue remains in doubt, the bladder may be opened longitudinally from an extraperitoneal approach (Fig. 23-6), and the trigone identified. Indigo carmine dye, 5-10 mL injected intravenously, should appear within minutes from both ureteral orifices. If ureteral integrity remains in question, an 8-French catheter may be threaded in a retrograde manner into the ureter under direct observation using a right-angle clamp. If injury is identified, intraoperative urologic consultation will allow the outcome to be optimized. It must be emphasized that there may be ureteral injury despite meticulous surgical technique, often as a result of an unexpected anatomic variation or distortion in the course of the ureter.
FIGURE 23-6. A. The bladder is opened extraperitoneally. B. The trigone is identified.
RETAINED PLACENTA
Following delivery of the infant, delivery of the placenta may be expected. If spontaneous delivery is delayed, manual extraction may be indicated. No data exist to document an ideal duration of the third stage of labor prior to a manual removal; however, in a recent review, Combs and Laros (1991) found no increase in hemorrhage until the third stage exceeded 30 minutes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree