KEY POINTS
• Teratogens are substances, organisms, or physical agents capable of causing abnormal fetal structural development, growth restriction, or death.
• Major defects are apparent at birth in about 3% of the general population.
• Many defects arise from multifactorial patterns of inheritance.
• The most common organ system for congenital abnormalities is the cardiovascular system.
• The incidence of neural tube defects (NTDs) and possibly other anomalies (particularly those associated with taking antiepileptic medications) may be significantly decreased by taking folic acid before conception.
BACKGROUND
Definitions
• A teratogen is a substance, an organism, or a physical agent capable of causing abnormal development. A teratogen can cause abnormalities of structure or function, intrauterine growth restriction, and death.
• Teratogenesis is a medical term from the Greek literally meaning “monster making.” The term has gained a more specific usage for the development of abnormal cells causing physical defects in the fetus.
• Teratology is the study of the frequency, causation, and development of congenital malformations.
• Congenital malformations and birth defects are terms that are often used interchangeably. Congenital malformations are fetal physical defects. Birth defects have evolved beyond the original emphasis on congenital malformations to denote a broader category of developmental abnormalities that includes physical defects.
Pathophysiology
• It was once believed that the mammalian embryo developed in an impervious uterus, protected from extrinsic factors. The thalidomide disaster of the 1960s made it apparent that the developing embryo is highly vulnerable to extrinsic agents that may have a negligible effect on maternal well-being.
• Teratogens come from many sources. Research is focusing on the possible causative actions of teratogenic agents to determine their mechanism, as well as the site of action.
• James G. Wilson (1) developed the six principles of teratology for the development in the refinement of the mammalian embryo. These principles are
• Susceptibility: Susceptibility to teratogenesis seems to depend on the genotype and the manner in which it interacts with environmental factors.
• Timing of exposure: Susceptibility to teratogenic agents varies with the developmental stage at the time of exposure.
• Mechanisms: Teratogenic agents act in specific ways on developing cells to initiate abnormal development in embryogenesis.
• Manifestations: The final manifestation of abnormal development may be malformation, intrauterine growth restriction, functional disorders, or death.
• Access of adverse environmental influences to developing tissue depends on the nature of the agent (route and degree of maternal teratogen exposure, rate of placental transfer, and systemic absorption).
• Dose effect: Manifestations of abnormal development increase as the dosage increases from no effect to lethal levels.
Etiology
Known causes of developmental malformations include (2)
• Genetic transmissions (Mendelian disorders, single gene): 15% to 20%
• Chromosomal abnormalities: 5% to 10%
• Aneuploidy (i.e., Down syndrome and Turner syndrome)
• Environmental causes
• Maternal infections: 1% to 3%
• Maternal conditions, including illicit substance abuse: 1% to 4%
• Deformations: 1% to 2%
• Drugs, chemicals, radiation, and hyperthermia: 1% to 2%
• Unknown: 65% (Table 30-1)
Table 30-1 Estimated Incidents of the Leading Categories of Birth Defects
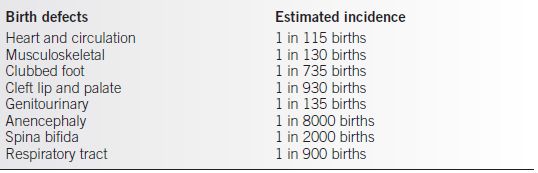
Data for this table are found in references 5 to 14.
TERATOGENS
Background
Definitions
• The U.S. Food and Drug Administration (FDA) oversees the safety of drugs and provides the most widely used system to grade the teratogenic effects of medications.
• The FDA assigns a safety category for the use of medications during pregnancy using a five-letter system, A, B, C, D, and X (3,4):
• Category A: Adequate and well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities.
• Category B: Animal studies have revealed no evidence of harm to the fetus; however, there are no adequate and well-controlled studies in pregnant women, or animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus.
• Category C: Animal studies have shown an adverse effect, and there are no adequate, well-controlled studies in pregnant women, or no animal studies have been conducted, and there are no adequate and well-controlled studies in pregnant women.
• Category D: Adequate, well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus; however, the benefits of the therapy may outweigh the potential risks.
• Category X: Adequate, well-controlled, or observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities. The use of the product is contraindicated in women who are or who may become pregnant.
• The safety category must be displayed on the labels of all drugs.
Etiology
• Major defects are apparent at birth in about 3% of the general population and in about 4.5% by 5 years of age. A cause or mechanism for the defect is typically determined in less than 50% of the cases (5,6).
• Health care providers and obstetricians are often asked about the potential teratogenic effect of various agents.
• Some medications rarely cause birth defects, and others commonly cause them.
• Some agents cause major defects if fetal exposure occurs during a specific critical period but cause no harmful effect at another time in gestation. After organogenesis has been completed (13 weeks of gestation), the observable effect of extrinsic agents may be limited to growth restriction rather than manifest as a defined structural abnormality.
• Individual differences exist in susceptibility to teratogenic effects from the same agent. Unfortunately, when counseling patients, it is impossible at the present time to know the individual’s genotypic sensitivity or resistance to a given agent. So the potential for abnormalities exists for each individual, although a wide spectrum of different outcomes can be anticipated.
• Animal studies have been used extensively to determine possible teratogenic effects, and the results have been used in counseling individuals and couples at risk. Although animal studies may be helpful in counseling, they do not reliably predict human effects. Specific teratogenic effects may be species specific. This was the case with thalidomide, where the teratogenic effect was not seen in animals (6,7).
Evaluation
• Prenatal testing should be provided when indicated by risk assessment or other findings.
• Some patients, once they are fully informed of the risk, may request pregnancy termination.
• Follow-up counseling is needed to provide emotional support, as well as education, regardless of whether the patient/couple chooses termination or continuation of the pregnancy.
• Multispecialty support for an abnormal infant is essential.
Diagnosis
• Prenatal diagnosis is available for many diseases and possible for many congenital anomalies that are linked to teratogenic agents. Unfortunately, not all abnormalities can be anticipated or detected, particularly those defects caused by environmental agents.
• Targeted ultrasound can be used to diagnose many structural and developmental abnormalities.
• Genetic amniocentesis provides access to amniotic fluid for karyotype determination, polymerase chain reaction for viral exposures, and assessment of specific markers such as α-fetoprotein (AFP).
• Chorionic villus sampling (CVS) and fetal blood sampling assist with prenatal diagnosis in specific situations.
• Confirmed or suspected agents are typically placed in one of three categories (4–13) (Table 30-2):
• Infectious agents
• Drugs and chemicals
• Physical agents
Table 30-2 Suspected Human Teratogens
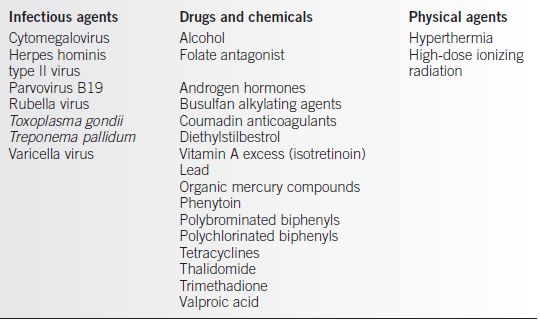
From Shepherd TH. Catalog of teratogenic agents. 7th ed. Baltimore: Johns Hopkins University Press, 1992. (See also Beckman and Brent (2).)
INFECTIOUS AGENTS
Cytomegalovirus
Diagnosis
Clinical Manifestations
• Hydrocephaly, microcephaly with cerebral calcifications
• Chorioretinitis
• Intrauterine growth restriction, typically early onset or symmetric
• Microphthalmia and hearing loss
• Severe mental retardation, developmental delay
Characteristics
• Cytomegalovirus is one of the most common congenital infections.
• The congenital infection rate is 40% after primary infections during pregnancy and possibly only 15% after recurrent infections.
• Of the infected infants, physical effects may only be present in 20% after a primary infection and 8% after a secondary infection.
• No apparent effective therapy exists (4,8).
Herpes
Diagnosis
Clinical Manifestations
• Intrauterine growth restriction
• Microcephaly with cerebellar necrosis
• Chorioretinitis, cataract, microphthalmia
• Hepatosplenomegaly
Characteristics
• Passage of virus can be transplacental or transcervical.
• Majority of newborn infections occur by contact with infected genital secretions.
• With primary infection near the time of delivery, there is a 33% to 50% risk of transmitting the virus to neonate with vaginal delivery.
• With recurrent disease, the risk of transmission may be 2% to 5%.
• Prevention of transmission is primarily by cesarean delivery for those at risk at delivery (8).
Parvovirus
Diagnosis
Clinical Manifestations
• Hydrops
• Ascites
• Placentomegaly
• Hypertrophic myocardiopathy
• Ventriculomegaly
Characteristics
• Transplacental transmission.
• Parvovirus B19 leads to fetal anemia by affecting the erythroid precursor cells. It also has an affinity for cardiac myocytes, resulting in fetal myocarditis.
• Risk of congenital infection from an infected mother is 10% to 20%.
• Management is by in utero transfusion. Fetal anemia can be predicted with Doppler sonography of the middle cerebral artery peak systolic velocity (8).
Rubella
Diagnosis
Clinical Manifestations
• Microcephaly
• Mental retardation
• Cataracts and deafness
• Congenital heart defects (8)
Characteristics
• Malformation rate may exceed 50% if the infection occurs during the first trimester.
• The rate of severe malformations decreases to less than 10% by midpregnancy.
• Immunization of children and nonpregnant adults is necessary for prevention.
• Immunization is not recommended during pregnancy.
• The live, attenuated vaccine virus has not been shown to cause congenital rubella malformations (8).
Toxoplasmosis
Diagnosis
Clinical Manifestations
• Microcephaly, hydrocephalus, and cerebral calcifications.
• Chorioretinitis.
• Severity of malformations apparently depends on the duration of the disease.
Clinical Characteristics
• Overall, in the United States, infection during pregnancy is rare, occurring in less than 0.5% of pregnant women.
• For the fetus to be at risk, the primary infection has to occur during pregnancy.
• Transmission is by raw meat or exposure to cat feces that are infected.
• In the first trimester, with maternal infection, the incidence of fetal infection is less than 10%, but this increases to greater than 50% in the third trimester.
• The incidence and severity of congenital infections or malformations are greatest with first-trimester fetal infection.
• Pyrimethamine sulfadiazine and spiramycin are the antibiotic treatments of choice (8).
Syphilis
Diagnosis
Clinical Manifestations
• Congenital syphilis confers a 50% risk of intrauterine fetal death or neonatal death.
• An infant’s symptoms may not be apparent until weeks or even years after birth.
• Early congenital syphilis is characterized by rhinitis, fever, pneumonia, skin problems, low birth weight, irritability, and hepatosplenomegaly.
• Skin rash is an early sign in the infant with congenital infection.
• Findings consistent with late congenital syphilis:
• Dentition: Hutchinson teeth
• Eye: Corneal scarring, chorioretinitis
• Ear: Eighth nerve deafness
• Nose: “Saddle nose”
• Central nervous system: Mental retardation, palsies/paresis
• Bones: Saber shins, Clutton joints
• Late maculopapular rash on face and palms
Characteristics
• Congenital syphilis is potentially a preventable disease.
• Maternal serologic testing early in pregnancy is the key to prevention.
• Antibiotic treatment is effective for maternal infection but may not correct established fetal infection (8).
Varicella
Diagnosis
Clinical Manifestations
• Potentially, all organs can be affected by varicella, though skin scarring is a characteristic abnormality. Extremity and limb abnormalities with muscle atrophy and hypoplasia of the hands and feet are common.
• Chorioretinitis and cataracts are characteristic in the newborn infant.
• Microcephaly may be present.
Characteristics
• The risk of congenital varicella appears to be linked to maternal infection between 7 and 21 weeks of gestation.
• A separate syndrome of potentially fatal neonatal infection can occur with maternal varicella infection within 5 to 7 days of delivery. Zoster immunoglobulin is available for newborns delivered under this circumstance.
• Varicella vaccine is available for nonimmune women before conception (8).
DRUGS AND CHEMICALS
Folic Acid Antagonist and Disruption of Folic Acid Metabolism
Diagnosis
Clinical Manifestations
• Increased risk of spontaneous abortions
• Multiple anomalies
Characteristics
• Exposure to folic acid antagonists during the first trimester carries a malformation incidence of more than 30%.
• Preconception identification and counseling of women on folic acid antagonists is the important step in preventing these malformations.
• Several congenital anomalies with examples of NTDs and cardiac defects may have an origin from disruption of folic acid metabolic pathways (9,10).
Genetic. Multifactorial anomalies are caused by interactions of environmental exposures by certain altered genes. An example is mutation of the gene for methylene tetrahydrofolate reductase. This genetic abnormality is associated with several malformations including the example of NTDs in women who have inadequate folic acid intakes.
Homeobox G. An example of homeobox gene creating teratogenicity is with retinoic acid. Retinoids such as vitamin A activate genes essential for normal growth and tissue development. Retinoic acid is a teratogen that can activate these genes prematurely. This mechanism has been linked to abnormalities in central nervous system development.
Anticonvulsant Medications
Background
A history of epilepsy presents an increased risk of fetal anomalies. It has been uncertain whether the risk is secondary to the underlying seizure disorder or to medication exposure (10). A meta-analysis of congenital malformation in women with epilepsy found an overall rate of 7.1% in pregnancies with epilepsy versus 2.2% in controls. Additionally, there are limited data available for the newer anticonvulsants:
• If possible, valproate and polytherapy should be avoided during pregnancy, particularly in the first trimester.
• With monotherapy, the malformation rates were
• Valproate 10.7%
• Phenytoin 7.3%
• Carbamazepine 4.6%
• Phenobarbital 4.9%
• Lamotrigine 2.9%
• With polytherapy, overall the rate was 16.8% as compared to polytherapy with valproate that demonstrated a rate of 25.0%.
• Choice of antiepilepsy drug preconception should be
• Most effective for seizure control
• Monotherapy if possible
• Least teratogenic
• Lowest dose
Phenytoin
Diagnosis
Clinical Manifestations
• Microcephaly with mental retardation
• Craniofacial features with dysmorphism
• Intrauterine growth restriction
• Cardiac defects
• Hypoplasia of the distal phalanges and nail beds
Characteristics
• The full syndrome is seen in less than 10% of offspring exposed in utero.
• Some clinical manifestations are seen in up to 30% of exposed fetuses/infants.
• Mild to moderate mental retardation is noted frequently. The effects may depend on whether the fetus inherits a mutant gene that decreases the production of epoxide hydrolase, the enzyme necessary to decrease this production.
• Some studies suggest the possibility that folic acid supplementation could decrease the incidence of some of these anomalies. The dosage of folic acid necessary to achieve this may be 4 mg daily, as compared to the 0.4 mg/d normally used for pregnancy supplementation (11,12).
Valproic Acid (Depakote)
Diagnosis
Clinical Manifestations
• NTDs, particularly open spina bifida
• Increased risk for heart defects and skeletal abnormalities
• Minor facial defects, including cleft lip and palate
Characteristics
• Valproic acid exposure before closure of the neural tube between the third and fifth weeks of gestation results in a 1% incidence of NTDs.
• There is evidence that high-dose folic acid supplementation during the critical exposure period decreases the risk of NTDs. The recommended supplementation dose is 4 mg daily, which is significantly greater than the FDA-recommended dose of 0.4 mg daily during pregnancy (11,12).
Carbamazepine (Tegretol)
Diagnosis
Clinical Manifestations
• Neural tube defects
• Craniofacial defects
• Microcephaly with developmental delay
• Fingernail hypoplasia
• Intrauterine growth restriction
Characteristics
• Risk of NTDs is 1% to 2% and requires exposure before neural tube closure at the third to fifth weeks of gestation. Concurrent use with other antiepileptic agents may increase the risk.
• As with other anticonvulsant drugs, high-dose folic acid supplementation may decrease the incidence of NTDs. The recommended dose is 4 mg daily before conception and during early pregnancy (11,12).
Trimethadione
Diagnosis
Clinical Manifestations
• Characteristic facial appearance with cleft lip/cleft palate and ophthalmologic abnormalities
• Cardiac defects
• Microcephaly with mental retardation
• Intrauterine growth restriction
• Limb defects
• Genitourinary abnormalities
Characteristics
• The risk for defects or fetal loss exceeds 60% with first-trimester exposure.
• A syndrome characteristic for exposure includes V-shaped eye brows, low-set ears, and high, arched palate (11,12).
Lamotrigine (Lamictal)
• Registries report 2.7% to 3.2% risk of major malformation, similar to controls.
• Early studies noted a higher than expected rate of facial clefts 8.9/1000 compared with 1–2/1000.
• Drug level markedly increased with valproate and decreased with hormonal contraceptives and pregnancy (65% increased clearance).
• Lamotrigine Pregnancy Registry: 800-336-2176 (11,12).
Levetiracetam (Keppra)
• Mechanism of action unknown
• No adequate, well-controlled studies in pregnant women
• Two registries are available:
• Antiepileptic Drug Pregnancy Registry 888-233-2334 www.mgh.harvard.edu/aed/
• UCB AED Pregnancy Registry 888-837-7734 (11,12)
Phenobarbital
• One of the oldest anticonvulsant medications still in use.
• Phenobarbital is a folic acid agonist.
• Major cardiac, craniofacial, genitourinary abnormality rates similar to other therapy (e.g., Tegretol, Dilantin).
• Risk of congenital anomalies appears greater among infants whose mothers required phenobarbital and phenytoin during pregnancy compared to infants of mothers treated with phenobarbital alone.
• Associated with sedation in mother and nursing infants.
• Phenobarbital-exposed newborns may exhibit a withdrawal syndrome characterized by hyperactivity and tremors.
• Chronic maternal phenobarbital therapy may induce a vitamin K responsive bleeding abnormality in the neonate.
Choice of Antiepileptic Drugs (AEDs) (11,12)
| First generation | Pregnancy category |
| Valproate (Depakote) | D |
| Carbamazepine (Tegretol) | D |
| Phenytoin (Dilantin) | D |
| Phenobarbital | D |
| Second generation | Pregnancy category |
| Lamotrigine (Lamictal) | C |
| Levetiracetam (Keppra) | C |
| Topiramate (Topamax) | C |
| Zonisamide (Zonegran) | C |
| Oxcarbazepine (Trileptal) | C |
Antimicrobials
Penicillin, ampicillin, and amoxicillin appear to be safe in pregnancy. Collaborative perinatal project did not show an increased risk of anomalies. It has been suggested that amoxicillin and clavulanate may lead to abnormal microbial colonization of the gastrointestinal tract leading to a potentially increased risk of necrotizing colitis. It is recommended that amoxicillin and clavulanate be avoided in women at risk for preterm delivery.
Aminoglycosides
• Both nephrotoxicity and ototoxicity have been reported in preterm newborns treated with gentamicin.
• Congenital defects from prenatal exposure have not been documented.
Cephalosporins
• There is no consensus of teratogenicity.
Sulfonamides
No teratogenic effects have been noted. Sulfonamides can compete with bilirubin for binding sites on albumin. This can raise free bilirubin in the serum increasing the risk of hyperbilirubinemia in the neonate (13).
Trimethoprim
There are no studies that substantiate teratogenicity.
Nitrofurantoin (Macrodantin) is capable of inducing hemolytic anemia in patients deficient in G-6-PD deficiency. However, no studies have shown an increase in hemolytic anemia in newborns as a result of intrauterine exposure (13).
Fluoroquinolones
• Quinolones (ciprofloxacin) may cause cartilage damage and arthropathies in children. Its use has been discouraged during pregnancy.
Metronidazole (Flagyl) studies have failed to show any increased incidence of congenital defects.
Topical Antifungal Agents
• Mycostatin (nystatin), clotrimazole (Lotrimin), or miconazole (Monistat)
• Not known to be associated with any congenital malformations
Antivirals
• Amantadine may be used in treatment or preventive measures for influenza. At very high doses in animal studies, it has been reported to be teratogenic. Several case studies suggest that when the medication has been given in the first trimester, it may have a possible increased risk of congenital heart defects, but this information is very limited.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


