CHAPTER 20 Surgical procedures for recovery of hand function
Summary box
Introduction
A brachial plexus injury (BPI) is a devastating injury, especially when it involves the lower roots with associated loss of hand function.1–4 In the developing world, these injuries are frequently caused by motorcycle accidents.5 BPI can be classified into an upper arm type (C5,6 with or without C7), a lower arm type (C8T1), and a total arm type (C5-8T1).6 Amongst these three types, hand function is mostly preserved in the upper arm type, except when there is involvement of the C7 root. However, hand function is severely compromised in both the lower arm type and the total arm type. The restoration of hand function is challenging because of the great distance between the proximal plexus injury and the neuromuscular endplate of the forearm and hand muscles (approximately 40–60 cm). Even if axon regeneration proceeds smoothly (1 mm/day), it would take more than 18 months to reach the target muscle, by which time irreversible atrophy of the muscle would have occurred. The results of early nerve reconstructive procedures (neurolysis, nerve grafting, and nerve transfers) for restoration of hand function are poor when compared to the results obtained at the shoulder or the elbow. Therefore secondary procedures like tendon and muscle transfers are frequently used to restore hand function.
Nerve reconstructive procedures
Neurolysis
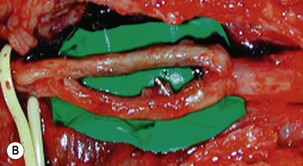
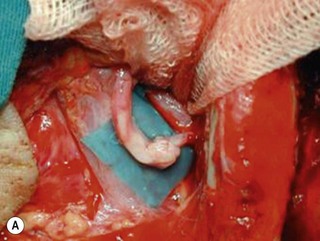
Microsurgical neurolysis of the brachial plexus is a controversial procedure with uncertain results. Lesions in continuity with external compression can be treated successfully by neurolysis.6 However, neurolysis is rarely effective in the recovery of hand function, except when a conduction block (neuropraxia) does not resolve spontaneously. When there is fibrosis of the perineurium and the epineurium, an incision over the nerve sheath can be performed. These surgical procedures can have a good result when the fascicular pattern and the endoneurial tissue are preserved (Figure 20.1). If there is fibrosis in the fascicles or a loss of the fascicular pattern, neurolysis will not be successful and resection of the involved segment and restoring nerve continuity by nerve grafting is indicated. In many cases, it is not easy to differentiate between a salvageable nerve that can be preserved and a damaged nerve that should be resected. In such situations, it is better to resect and graft than to attempt neurolysis.
Primary suture
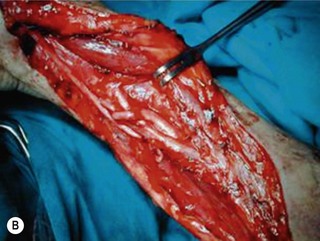
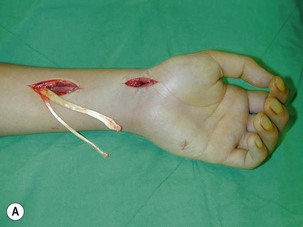
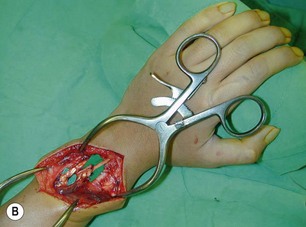
Direct neurorrhaphy is a successful treatment for a cleanly transected nerve, such as with a stab wound. In a typical brachial plexus avulsion lesion with loss of nerve substance, a direct neurorrhaphy is not possible and should not be attempted (Figure 20.2). Narakas reported a large series of 800 BPI cases that were treated surgically. Primary suture was possible in only 21 cases (5 incised wounds and 16 iatrogenic injuries).7
Most BPI cases today are caused by high-energy trauma with nerve avulsion injuries that are not suitable for primary repair. In a reported series of 386 cases of surgical procedures for BPI, only 5 primary repairs were performed.8 In patients with a partial nerve laceration after a stab or an incised wound, it is relatively easy to reconstitute the general fascicular organization of the nerve trunk by end-to-end suture, and the prognosis is usually good. The primary suture of a transected nerve trunk/cord should be performed immediately to avoid neuroma formation and the difficulty associated with intraneural dissection (Figure 20.3). With regard to C8T1 lesions of lower arm-type BPI, primary suturing has not resulted in satisfactory outcomes of hand function and additional procedures such as tendon grafting or free functional muscle transfers are always indicated.9
Nerve grafts
The results of nerve grafting to improve hand function in BPI patients are not satisfactory. In the total arm-type BPI, it is rare to obtain usable function in the hand by nerve graft surgery. In the majority of cases, patients merely obtain a “shovel hand” or “paperweight hand,” which is only useful to stabilize an object on a table.10,11 For patients with lower arm-type BPI, decisions regarding surgery should be based on the clinical examinations and additional diagnostic studies. When pseudomeningoceles appear on the preoperative magnetic resonance imaging or myelography studies, nerve graft procedures should not be performed because outcomes are invariably poor.12 In this situation, muscle/tendon transfer or a free functioning nerve transfer (FFMT) should be considered to reconstruct the hand. However, if the magnetic resonance imaging or myelography studies are normal in lower arm-type BPI cases, but spontaneous regeneration (by electromyogram and nerve conduction velocity) has not occurred, exploration surgery over the supraclavicular region is appropriate to assess the suitability of using nerve grafts. The early use of nerve grafts in such cases has restored extrinsic hand function in the M2–M3 range, but intrinsic hand function cannot be recovered (Figure 20.4).13
Neurotization for motor functions
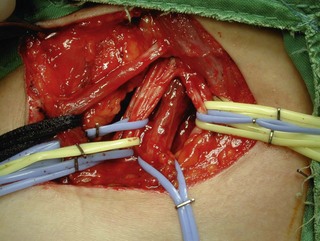
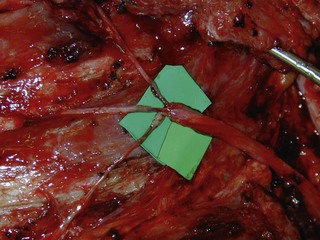
The distal nerve stump may be reinnervated either by another root within the plexus (intraplexal neurotization) or by a healthy nerve that resides outside the plexus (extraplexal neurotization). It is almost impossible to gain useful hand function by neurotization because of the distance involved and limited donor proximal nerves.9 In addition, axon count in extraplexal motors is significantly lower when compared to the normal nerve roots. An intraplexal transfer, proposed by Chuang, uses a vascularized ulnar nerve graft to bridge the gap between an ipsilateral C5 or C6 root and the median nerve in patients with total arm-type BPI (C5 and/or C6 rupture associated with C7,8T1 three-root or C6-8T1 four-root avulsion).14 The transfer of ICN’s to the median nerve to restore hand function is an extraplexal transfer (Figures 20.5 and 20.6).15,16
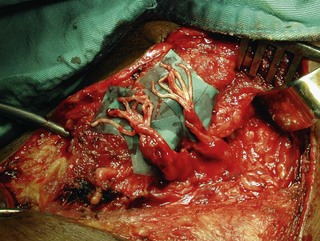
To overcome the limited number of available donor nerves and insufficient myelinated axon fibers in the donor nerves, Gu from China proposed using the contralateral C7 root to achieve useful hand function.17,18 This involves two stages. In the first stage, the pedicled ulnar nerve graft raised on the paralyzed side is coapted to the contralateral C7 nerve root. The second stage is done 8–12 months later and the distal end of the reinnervated ulnar nerve graft is sutured to the avulsed plexus, giving priority to the musculocutaneous nerve, the median nerve, the axillary nerve, and then the other nerves (Figures 20.7 and 20.8). Chuang prefers to transfer the ulnar nerve with the ulnar artery as a free flap, a procedure he calls the supercharged contralateral C7.19 In Chuang’s series, 8 out of 15 patients needed a secondary procedure with FFMT for hand function.20 Chuang also noted that sectioning of the C7 root created a temporary weakness of the triceps and paresthesia in the superficial radial nerve territory over the dorsum of the hand in the donor upper limb.
A sound knowledge of the anatomy of the C7 root, and muscles innervated by it is essential before considering this transfer. A transection of the posterior division of C7 produces a weakness of the radial nerve-innervated muscles, especially the triceps and the wrist and finger extensors; however, there is no paralysis. These muscles regain their original strength by internal sprouting. The transection of the anterior division of C7 slightly weakens the pectoralis muscle without visible loss of function and results in some sensory loss in the thumb and index finger. This sensory loss may not be tolerated well by some patients. In order to decrease the morbidity of C7 transfer, Millesi emphasized that a contralateral C7 transfer should never be performed immediately.6 Instead, Millesi recommends putting ligatures over the anterior and posterior divisions of the C7 root, and then examining the patient carefully the next day. This would allow assessment of loss of function. A major loss of sensory function would indicate that the anterior division should not be cut and only the posterior division should be used. If there is significant loss of motor function, the C7 transfer is not performed at all.
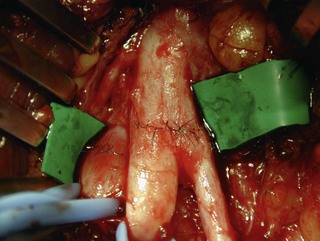
Waikakul et al., in their review of the results of using the anterior portion of the contralateral C7 to the median nerve for total arm-type BPI, got wrist flexion (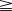 M3) in 29%, and finger flexion (
M3) in 29%, and finger flexion (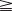 M3) in 21% of cases.21 In Songcharoen’s series, only 29% of patients achieved M3 or M4 finger flexion by contralateral C7 transfer.5 It is obvious that hand function in total arm-type BPI patients could not be reconstructed satisfactorily by only a contralateral C7 to median nerve transfer (Figure 20.9). The failure of contralateral C7 transfers is primarily due to the long distance between the C7 donor nerve coaptation site and the hand muscles innervated by the median nerve.
M3) in 21% of cases.21 In Songcharoen’s series, only 29% of patients achieved M3 or M4 finger flexion by contralateral C7 transfer.5 It is obvious that hand function in total arm-type BPI patients could not be reconstructed satisfactorily by only a contralateral C7 to median nerve transfer (Figure 20.9). The failure of contralateral C7 transfers is primarily due to the long distance between the C7 donor nerve coaptation site and the hand muscles innervated by the median nerve.
A new method of neurotization to restore hand function has been proposed. This requires a harvest of the full length of the phrenic nerve (via a thoracotomy) that is transferred to the median nerve in the distal arm.22,23 Zhao et al. performed an anatomical study on 17 cadavers and reported clinical application in 1 patient.22 They found that the branches of the median nerve in the distal arm were consistently organized into three fascicular groups located in the anterior, middle, and posterior parts of the median nerve trunk. The anterior fascicular group was composed of the branches to the pronator teres and the flexor carpi radialis. The posterior fascicular group was composed of the anterior interosseous nerve and branches to the palmaris longus, whereas the middle fascicular group was made up of the branches to the hand and the flexor digitorum superficialis (FDS). They reported a patient with total arm-type BPI where they transferred the phrenic nerve to the posterior fascicular group of the median nerve. The patient obtained M4–M5 recovery of flexor pollicis longus and the flexor digitorum profundus (FDP) of all four digits at 16 months after surgery. The harvest of the full length of the phrenic nerve allowed transfer to the level of the distal arm, thus shortening the distance and the time needed for reinnervation of the forearm muscles. This technique, although quite promising, has not gained popularity in BPI reconstruction. Further studies and additional clinical cases are needed to assess hand motor recovery and donor site morbidity, such as pulmonary function deterioration after phrenic nerve harvest.
Neurotization for sensory function
Most reconstructive procedures for total arm-type BPI have focused on the restoration of motor function, and few studies have addressed restoration of sensory function. The lack of cutaneous perception on the reconstructed hand will inevitably affect the prehensile function of the hand, such as lifting or holding objects in daily activities, because of a lack of tactile sensory feedback.24 Furthermore, a reconstructed hand with motor recovery, but without sensory restoration, will not give the patient protective sensation. Several types of sensory reconstructive procedures have been reported in the literature, such as primary nerve grafting on the trunk level, and intraplexal or extraplexal neurotization.2,25,26
Three different types of nerve transfer techniques have been described using C5 nerve root, ICN, or the supraclavicular nerve as the donor nerve.24 When C5 was used as the donor nerve, a free vascularized nerve graft from the sural nerve or from the ulnar nerve in the paralyzed forearm was harvested, and then transferred to the lateral root of the median nerve at the infraclavicular level of the affected side. When the ICN was used as the donor nerve, the lateral cutaneous branches of the third through sixth ICN were assembled with fibrin glue, and then directly transferred to the median nerve around the axillary region. Kotani et al. reported the results of 15 cases in which the ICN had been used for sensory reconstruction.27 Limited sensation of touch and pain with paresthesia were obtained in 11 cases. In 1977, Millesi reported the recovery of protective sensation in 15 of 18 cases who received ICN nerve transfers to the median nerve for total arm-type BPI.26 Hattori et al. reported 17 cases receiving ICN transfer to either the median nerve or the ulnar nerve for sensory reconstruction of total avulsion BPI. All of their patients obtained protective sensation of the hands, and perceived at least the 6.65 filament in the median or ulnar nerve territories. Eight patients perceived heat sensation and 13 patients perceived cold sensation, but none regained two-point discrimination.28
When the supraclavicular nerve was used as the donor nerve, two or three branches of this nerve were assembled with fibrin glue, and then directly sutured to the median nerve component of the lateral cord at the clavicular level without nerve graft. They reported an acceptable sensory recovery in 12 of 15 patients, and concluded that even the limited sensory recovery was useful for completely anesthetic BPI patients.24 The contralateral C7 transfer has been reported to have satisfactory sensory recovery. Although this method was originally designed for reconstructing motor functions, reports in the literature suggest that sensory recovery was better than motor recovery.5,19,21,29,30 The reason for poor recovery of motor function following contralateral C7 transfer is muscle atrophy. However the sensory end-organs are preserved for much longer and, despite the long distance between the C7 donor nerve coaptation site and the docking site of the vascularized ulnar nerve coaptation with the median nerve, sensory reinnervation is usually satisfactory.
Tendon and muscle transfers
Tendon transfers
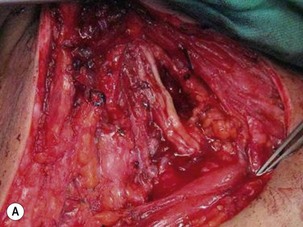
Tendon transfer techniques are useful for restoring hand function in patients with incomplete injuries or those who have partial recovery of function following nerve grafting or nerve transfer procedures.14,29 It should be borne in mind that any tendon that is transferred for another function will lose one grade of power after the transfer. For example, a M4 muscle will become a M3 muscle after transfer.
In upper-arm-type BPI with associated C7 injury, patients usually present with loss of wrist and finger extension. The functional needs of the hand after a C7 injury (radial nerve palsy) are: (1) wrist extension; (2) finger and thumb extension; and (3) thumb proximal stability.31 For wrist extension, a transfer of the pronator teres to the wrist extensors (extensor carpi radialis longus and extensor carpi radialis brevis) can be considered if there has been adequate recovery of pronator teres function. For finger extension and thumb extension, the traditional transfers are the flexor carpi ulnaris (FCU) to the extensor digitorum communis (EDC), and the palmaris longus to the extensor pollicis longus (EPL) are recommended. This FCU transfer often results in a slight radial deviation of the hand at the wrist. If the patient has significant radial deviation at the wrist, the insertion of the extensor carpi radialis longus should be transferred to the extensor carpi ulnaris, or the FCU tendon transfer should not be done. The finger and thumb extension can also be reconstructed by transferring the flexor FDS tendons to the long and ring fingers. The ring-finger FDS is attached to the EDC, similar to the FCU transfer, and the long-finger FDS is attached to the EPL. An alternative method is to transfer the palmaris longus to EPL, and the flexor carpi radialis to the EDC (Figure 20.10).
Tenodesis
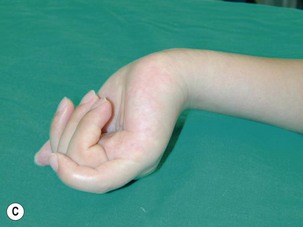
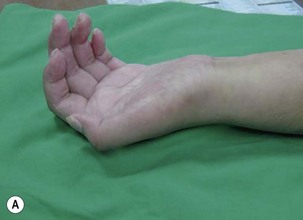
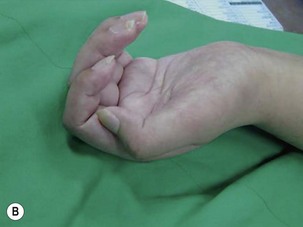
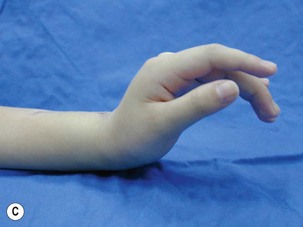
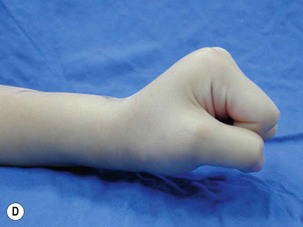
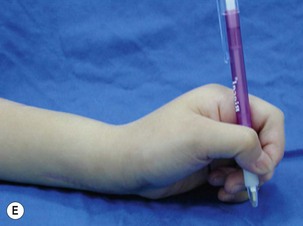
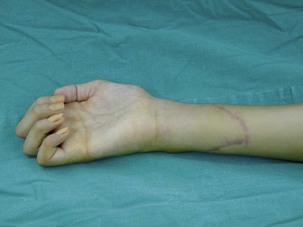
When a single FFMT is used to restore elbow and digital flexion, wrist and digital extension is usually not reconstructed and most of the reconstructed fingers are in a state of flexion contracture (Figure 20.11). Although some dynamic splintage devices have been developed to prevent flexion contractures of the fingers and wrist, tenodesis of wrist and finger extensor tendons is a useful procedure in these cases. The tenodesis procedures are always performed after the FFMT has recovered motor function. The position and angle of the wrist and hand in the tenodesis procedure depends on the degree of flexion deformity and the tightness of the soft tissue. A tenodesis on antagonist tendons may also enhance the efficiency of a previously transferred muscle.1,32,33
Muscle transfers
Pedicled latissimus dorsi muscle flap transfer
For the past 30 years, the latissimus dorsi muscle has been used to restore elbow flexion,34 but it is not often used to restore digital flexion. The restriction in the use of the latissimus dorsi muscle may be related to inadequate perfusion of the distal third of the muscle by the dominant vessel – the thoracodorsal artery.35 The perfusion of this portion of the muscle relies on the perforating branches of the intercostal and other collateral arteries. However, in the series published by Gousheh et al., the thoracodorsal artery was sufficient in nourishing the entire length of the latissimus dorsi muscle, and the pedicled latissimus dorsi muscle could be harvested for more than 40 cm, which is long enough to reach the target tendons over the forearm and wrist.32,36–38
Indications
The indications for pedicled latissimus dorsi muscle transfer in BPI include:
Surgical procedure
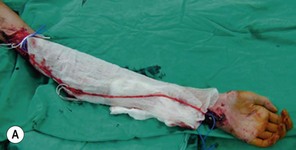
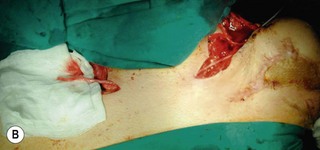
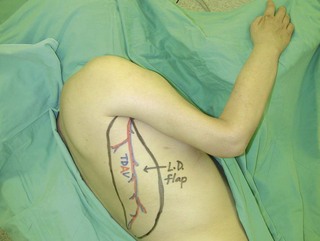
An incision running from the posterior axillary fold to the midpoint of the iliac crest is made (Figures 20.12 and 20.13). The skin is sutured to the muscle to prevent possible shearing of the cutaneous component of the flap because the skin is supplied by the perforating vessels coming through the muscle (Figure 20.14). The muscle is freed from its lateral, distal, and medial borders. A longer length of the muscle can be obtained by including a small portion of the gluteal aponeurosis or the fascia attaching the muscle to the spinous process along with the distalmost portion of the muscle. The flap is folded over, and its anterior surface is separated from the chest wall. The neurovascular pedicle (thoracodorsal artery, vein, and nerve) is identified and carefully dissected as far proximally as the circumflex scapular vessels to maximize the reach of the muscle flap. The branches to the serratus anterior muscle are ligated. This will enable the muscle to reach the forearm (Figure 20.15). At this point, the entire muscle flap can be lifted and the neurovascular pedicle on the upper part of this muscle is revealed. It is not necessary to divide the muscle insertion on the humerus as the limiting factor to movement is the vascular pedicle. However, dividing the muscle insertion from the humeral intertubercular sulcus and reattaching it to the acromion (a bipolar transfer) will give it a better biomechanical advantage as it places the muscle in a straight line over the arm and forearm. If this latissimus dorsi flap is being used for functional reconstruction of total arm-type BPI, two ICNs can be harvested at the same time, and neurotization is performed by coapting the two ICNs to the thoracodorsal nerve.
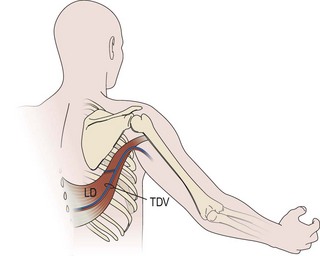
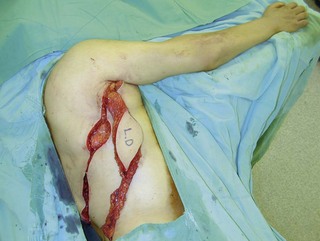
Figure 20.13 Latissimus dorsi muscle flap harvesting. LD, Latissimus dorsi; TDV, thoracodorsal vessel.
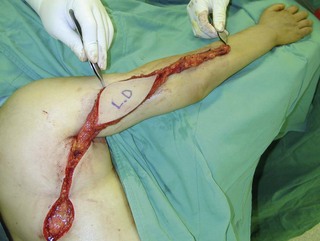
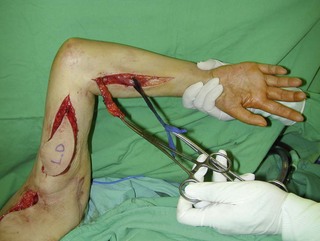
Figure 20.15 The length of the pedicle latissimus dorsi flap could easily reach to the right mid-forearm.
A subcutaneous tunnel is created between the axilla and the forearm and the muscle is carefully delivered through the tunnel, taking care not to twist the vascular pedicle (Figure 20.16). Pronator teres or the wrist flexors may serve as a pulley for finger flexion reconstruction, whereas brachioradialis or wrist extensors may serve as a pulley when the latissimus dorsi is used to reconst the finger extensors (Figure 20.17). The use of a pulley prevents the development of a bowstring deformity at the elbow. The distal fascial portion of the latissimus dorsi muscle is sutured to the FDP (Figure 20.18) or the EDC. The fingers are maintained either in a flexed position (for digital flexor reconstruction) by temporary, multiple Kirschner wire fixation or in an extended posture (for digital extensor reconstruction) by postoperative splinting/casting. It is recommended to keep the elbow at 90° flexion, when suturing the latissimus dorsi to the tendons. The wound over the donor site is sutured in layers and closed suction drainage is inserted. Active and passive range-of-motion finger exercises are started after K-wire or cast removal 3–4 weeks after surgery.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree