CHAPTER 10 Shoulder sequelae in children with brachial plexus palsy
Summary box
Introduction
Despite advances in prenatal and obstetrical care, the estimated prevalence of brachial plexus palsy remains between 0.1% and 0.4% of live births.1,2 The majority of children demonstrate spontaneous recovery; however, some will have persistent deficits. Children with incomplete recovery following brachial plexus palsy may have functional limitations because of muscle weakness and soft tissue contractures. Deformities vary in extent and severity, depending, in part, on the type of lesion and the degree of recovery. Persistent muscle weakness and imbalance affect both bone growth and joint development. Long standing muscle imbalance may result in progressive glenohumeral deformity.3,4
Clinical examination
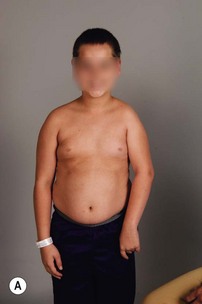
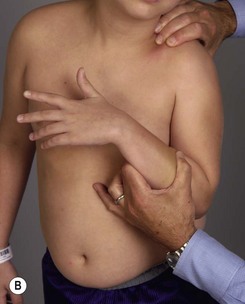
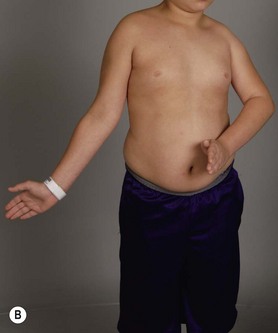
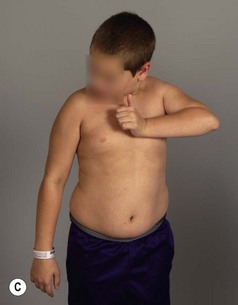
Physical examination remains the mainstay of analysis and decision making for children with brachial plexus palsy (BPP). Assessing shoulder function involves observation of spontaneous activity and stimulated activity with and without gravity assistance. Passive range of motion (ROM) of the shoulder and elbow should be assessed. Internal and external rotation is performed in both adduction and abduction (90 degrees) while stabilizing the scapula against the thorax (Figure 10.1).
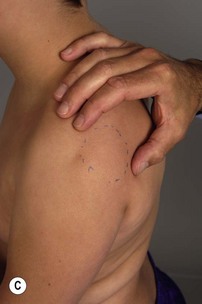
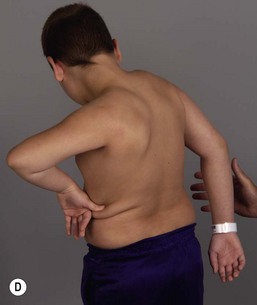
It is important to palpate for posterior humeral head dislocation. If the shoulder rests in a dislocated position, external rotation will be limited, but this will improve considerably with reduction of the humeral head into the glenoid. Internal rotation contractures and glenohumeral deformity have been described in children as young as 5 months of age.5 Infantile dislocations of the shoulder have also been reported. Moukoko et al.6 described posterior shoulder subluxation/dislocation in neonates and infants with BPP. Mean age at the time of diagnosis was 6 months (range, 3–10 months). Clinical signs included asymmetry of the skin folds in the axilla, apparent shortening of the humeral segment, and a palpable fullness of the shoulder posteriorly (Figure 10.2). There was no correlation noted between the occurrence of subluxation/dislocation and the initial neurologic deficit. The most salient clinical finding was the loss of passive external rotation between examinations, which indicated a posterior shoulder subluxation/dislocation.6
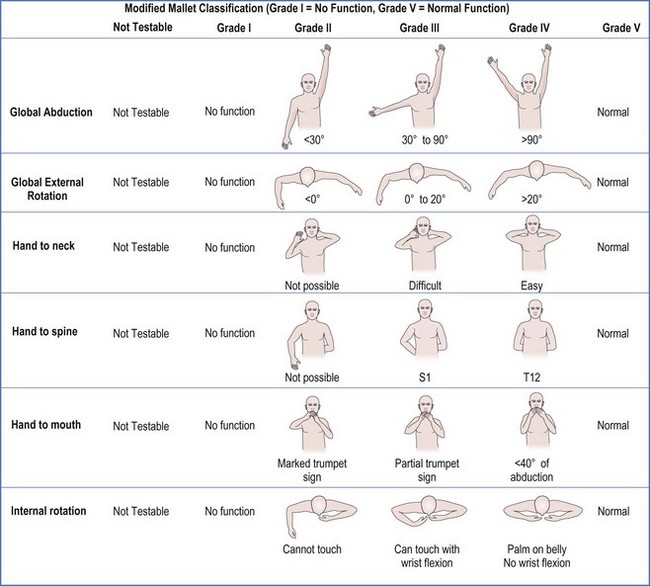
Recovery of muscle strength in children is graded by the Mallet Classification or Hospital for Sick Children scores. Fear of examination, lack of coordination, and inability to comprehend directions by the children challenge the examiner to assess active ROM and strength in the young child. The Hospital for Sick Children in Toronto, Canada developed the Active Movement Scale (AMS) as an alternative to the muscle grading system commonly used for adults.7 However, when contractures are present, this system is a limited tool because grades of strength may not sufficiently represent motor function.
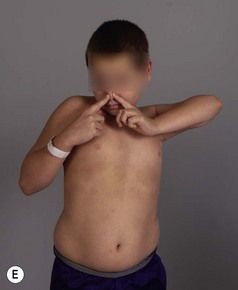
The modified Mallet scale is commonly used to assess shoulder function. A scale of 1 to 5 is used to evaluate shoulder abduction, global external rotation, and hand to neck, hand to back, and hand to mouth positions (Figure 10.3). An aggregate score is calculated by summing the 5 individual scores, with a maximum score of 25. Regarding internal rotation, we recognized that many children with residual BPP can not perform hand to spine prior to surgery (grade 1 or 2 Mallet score), but can perform midline tasks. Therefore, measurements of hand to spine may not detect loss of midline function, such as buttoning, zippering, and toileting. For this reason, we added a measure of midline function (a sixth subscale to the Mallet) to better assess loss of internal rotation and function (Figure 10.4).8 This additional measure balances the Mallet, which is weighted toward external rotation. The standard Mallet scores 3 activities of external rotation (external rotation, hand to neck, and hand to mouth) and only one of internal rotation (hand to spine). Measuring midline function decreases the bias toward external rotation and provides an assessment of midline activity, which is important for activities of daily living. Although no perfect scale exists that can be applied to all children, these tools, used in conjunction with measures of active and passive ROM of the upper extremity, aid the physician in making treatment decisions.
Imaging
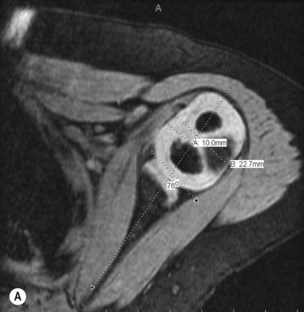
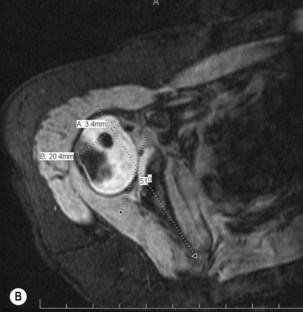
In the first few years of life the humeral head and glenoid are mostly unossified cartilage, limiting identification of glenohumeral pathology on plain radiographs. Ultrasonography, shoulder arthrography, computed tomography (CT), and magnetic resonance imaging (MRI) have all been used to better understand the morphology of the glenohumeral joint in children with BPP. Ultrasound has been used to identify normal relationships between the humeral head and glenoid, making the distinction between congruous and incongruous shoulders.9 Arthrography has been used to image the shoulder in the abducted position, and an axillary image can be particularly useful. Shoulder joint morphology was evaluated by Kon et al.10 in children with BPP undergoing surgical intervention for residual paralysis of the shoulder. Using arthrography, the position of the humeral head relative to the glenoid and the shape of the glenoid were evaluated on axillary views. They classified the glenohumeral joint into one of four categories: (1) concentric (2) flat (3) biconcave and (4) pseudoglenoid. The degree of shoulder joint deformity can also be quantified and graded using CT or MRI.11 MRI appears to offer the best detail of the glenohumeral joint; however, cost and need for general anesthesia in young children may limit the routine use of MRI (Figure 10.5).
Waters et al.12 proposed a classification system that graded the severity of glenohumeral deformity primarily based on the anteroposterior relationship of the humeral head and the glenoid. They found a significant relationship between increasing age and progressive glenohumeral deformity.12 Various other classification systems have also been proposed.13,14 These classifications show that with increasing deformity there is increasing posterior displacement of the humeral head from its normal concentric position, and the concave shape of the glenoid becomes increasingly convex. Pearl et al.15 found that there was strong agreement between arthrographic and MRI findings in patients with BPP and stressed the importance of preoperative imaging for all children with shoulder contractures to document the preoperative morphology of the glenohumeral joint, and for longitudinal follow-up of these patients postoperatively. This diagnostic information should be used in conjunction with physical examination to determine a surgical plan for the patient.
Management of residual deformities
The management of residual deformities of the shoulder remains controversial. The selection of procedures depends on a number of factors, including (1) the congruency of the shoulder; (2) the residual remodeling potential of the immature skeleton; (3) the strength of active muscles; and (4) the goals and concerns of the child and family. Zancolli16 classified residual deformities of the shoulder into several main types. The first general category includes contractures with internal rotation contracture of the shoulder being the most common in children with incomplete recovery. The internal rotation contracture results from an imbalance between the strength of the relatively unaffected internal rotators, which are the latissumus dorsi, subscapularis, and teres major and teres minor and the paralytic external rotators (infraspinatus).
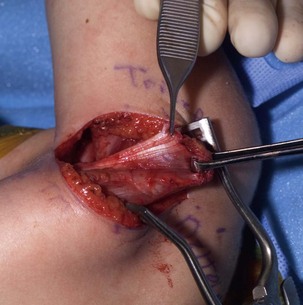
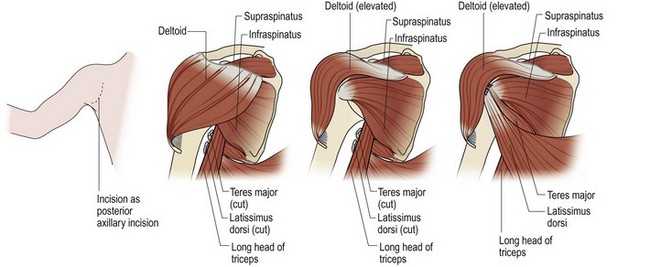
The initial goal in the infant is to maintain ROM and to keep the shoulder reduced during the period of reinnervation. If the joint remains congruous, soft tissue procedures to rebalance and reduce the joint are indicated. A variety of operations to address internal rotation contractures have been described in the literature. Essentially, each approach combines an internal rotation contracture release with or without a muscle transfer to augment external rotation. Fairbanks17 described the first formal anterior approach to the shoulder with release of the tight subscapularis and pectoralis and anterior capsule of the shoulder. L’Episcipo18 added a transfer of the teres major to an external rotator position, and Zachary19 added the latissimus to the external rotation transfer. Lengthening of the subscapularis and perctoralis with transfer of the latissimus and teres major was also the procedure used by Green and Tachdjian.20 Hoffer et al.21 modified the distal insertion of the latissimus and teres major to the rotator cuff. Ingram22 believed that lengthening of the subscapularis at the insertion potentially contributed to anterior instability and therefore described lengthening the subscapularis at its origin on the anterior surface of the scapula and moving the insertion of the teres major transfer proximally to the infraspinatus tendon. Hoffer and colleagues21 used the latissimus and teres major transfer into the rotator cuff insertion of the supraspinatus to enhance both external rotation and abduction (Figure 10.6).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree