Surgical Principles in Gynecologic Oncology
DENNIS S. CHI  ROBERT E. BRISTOW
ROBERT E. BRISTOW  DAVID CIBULA
DAVID CIBULA
The management of most human cancers involves multimodal therapy, and this is especially true for female genital malignancies. Although some early gynecologic cancers can be eradicated by surgery alone, and chemotherapy as a single modality can often cure gestational trophoblastic malignancies, the optimal treatment for the majority of gynecologic malignancies requires surgery combined with chemotherapy and/or irradiation.
In this chapter, surgery is discussed both as a separate discipline and as an integral part of multimodal therapeutic planning. Although specific operations and relatively new surgical techniques are described for some disease sites, many procedures are addressed more completely in surgical texts and atlases to which the reader is referred (1–3). The role of surgical intervention in the treatment of gynecologic cancers is addressed herein with a more philosophic approach than would be taken in a surgical atlas. However, select illustrations and tips are included that have enabled us to approach various radical procedures more confidently and safely. The major goal of this chapter is to give the reader an appreciation and understanding of the surgical principles of the subspecialty of gynecologic oncology.
TECHNICAL ASPECTS OF SURGERY
Many physicians who practice the art and science of surgery tend to focus on the technical craft of the specialty only when teaching young surgeons. At other times, we are acutely aware that the most difficult part of our practice is the decision of whether or not to utilize surgical therapy. Preoperative and postoperative management is also demanding since therapy must be tailored for individual patients depending not only on their specific disease but also on their overall medical status. Owing to the significance of these pressing issues, we often take for granted the many years of preparation and experience in the craft of surgery.
Mastering the skills of surgery involves a thorough understanding of proper technique. It also requires frequent and consistent practice to keep surgical maneuvers well honed. For the student, this means actual practice in tying knots, manipulating instruments, and suturing. For the accomplished surgeon, it means that a sufficient case load must be maintained to ensure adequate practice of the technical art of the specialty. The surgeon should also keep abreast of new suture materials, instruments, and technical developments.
Anatomy
There is no substitute for a detailed knowledge of the anatomy of the pelvis and abdomen. The physician who pursues gynecologic oncology as a career must be completely familiar with the pelvis, abdomen, retroperitoneum, and the lymphatic drainage of the female genital tract. No amount of surgical skill or knowledge of cancer therapy can compensate for the lack of this knowledge.
Lymphatic drainage from the cervix follows the uterine arteries and cardinal ligaments to the pelvic lymph nodes that include the external iliac, internal iliac (hypogastric), and obturator node groups (Fig. 9.1). From these pelvic lymph nodes, the drainage proceeds superiorly through the common iliac and presacral lymph nodes and then up to the paraaortic nodes.
The lymphatic drainage from both the uterine corpus and the ovaries follows one of three routes (Fig. 9.1): (a) along the uterine arteries in the broad ligaments to the pelvic nodes, (b) in channels following the round ligaments to the inguinal lymph nodes, or (c) along the ovarian lymphatics in the infundibulopelvic ligaments directly up to the paraaortic nodes.
The anatomy of the paraaortic lymph nodes has been well described by Fowler and Johnson (4). The paraaortic lymph nodes are part of the lumbar lymph node group. Usually, six subgroups of retroperitoneal nodes in the paraaortic region are recognized: paracaval, retrocaval, interaorto-caval, preaortic, paraaortic (left side), and retroaortic. The preaortic group drains the abdominal part of the gastrointestinal tract down to the mid rectum, whereas the retrocaval and retroaortic groups have no special area of drainage. The paracaval, interaorto-caval, and paraaortic (left side) nodes receive lymphatic drainage from the iliac lymph nodes, ovaries, and other pelvic viscera (apart from the alimentary tract), and therefore it is these groups of nodes that are sampled in the surgical staging of gynecologic malignancies. However, systematic paraaortic lymphadenectomy should address all major regions, including paracaval, retrocaval, interaorto-caval, preaortic, paraaortic, and retroaortic nodes.
There are typically 15 to 20 paracaval and paraaortic nodes per side. They are located adjacent to the inferior vena cava (IVC) and aorta, anterior to the lumbar spine, extending bilaterally to the medial margins of the psoas major muscles, and up to the diaphragmatic crura (4). The paracaval and paraaortic nodes usually dissected in gynecologic oncology span the region from the aortic bifurcation up to either the inferior mesenteric artery (IMA) or the renal veins.
The first major blood vessel encountered during a caudad-to-cephalad paraaortic node dissection is the IMA (Fig. 9.1). The IMA originates from the anterior surface of the aorta, approximately 3 to 4 cm above the aortic bifurcation. Next, the right and left ovarian arteries arise from their respective sides of the aorta, about 5 to 6 cm above the bifurcation (Fig. 9.1). The right ovarian vein inserts into the right side of the inferior vena cava (IVC), approximately 1 cm below the right renal vein. The left ovarian vein does not insert directly into the IVC, rather follows a path close to the left ureter inserting into the left renal vein lateral to the left border of the aorta. Three to four pairs of lumbar arteries and veins arise from the posterior surfaces of the aorta and IVC, respectively.
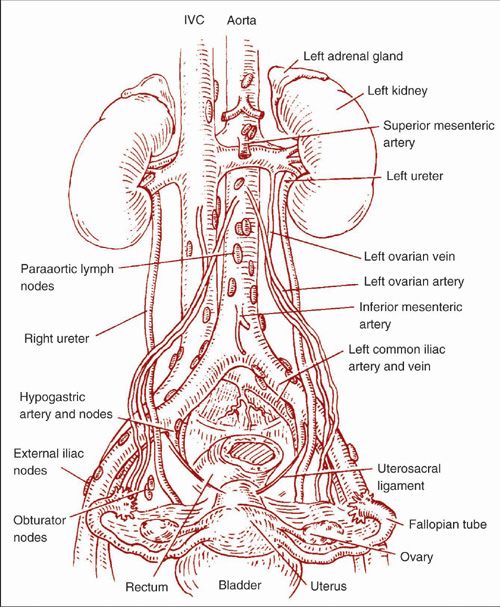
FIGURE 9.1. The pelvic and paraaortic lymph nodes and their relationship to the major retroperitoneal vessels.
The gynecologic oncologist who operates on patients with advanced ovarian carcinoma often encounters disease being spread across upper abdominal structures such as the diaphragm, liver, pancreas, and spleen. Debulking of tumor from these areas has been demonstrated to improve the rate of optimal cytoreduction and subsequent survival (5,6). Anatomic considerations for diaphragm surgery include the relevant hepatic attachments and the underlying central vasculature (7,8) (Fig. 9.2). The anterior hepatic attachment is the falciform ligament, which contains the ligamentum teres in its infrahepatic portion, and attaches the liver to the anterior abdominal wall in its membranous hepatic portion. As the falciform ligament continues superiorly, its peritoneal surface divides laterally on each side to form the anterior right and left coronary ligaments. These coronary ligaments reflect off the liver capsule and delineate the posterior extent of peritoneum covering the superior diaphragm. The IVC lies to the right side of this falciform ligament division. The right and left hepatic veins drain into the anterior surface of the IVC at the level of this peritoneal reflection. The anterior coronary ligaments continue laterally and inferiorly along the posterior liver edge, where they join the posterior right and left coronary ligaments to form the right and left triangular ligaments, respectively. The right triangular ligament reflects from the liver to the diaphragm, right kidney, and right adrenal gland. The left triangular ligament reflects primarily to the diaphragm; the posterior left coronary ligament lies higher than the esophageal hiatus and the esophagus is generally not encountered. The coronary ligaments on each side delineate the larger “bare area” of the liver on the right and a smaller “bare area” on the left, which underlie the central tendon of the diaphragm. The right phrenic nerve penetrates this central tendon lateral to the vena caval foramen on the right and is usually not encountered until the “bare area” is exposed. The left phrenic nerve may penetrate the left diaphragm muscle above the central tendon and is a consideration during left-sided anterior diaphragm surgery.
In the left upper quadrant of the abdomen, the spleen lies under the 9th, 10th, and 11th ribs. It is situated adjacent and slightly deep to the stomach and colon, lateral to the pancreas, and sits on the superior aspect of the left kidney. The posterior aspect of the spleen is in contact with the adrenal gland as well as Gerota’s fascia of the kidney. The tail of the pancreas often approaches the splenic hilum and sometimes contacts the spleen. The spleen varies in size between individuals, but in general measures 12 cm in length, 7 cm in width, and 3 to 4 cm in depth. The peritoneum creates folds that form the suspensory ligaments of the spleen. The four main “suspensory” ligaments are gastrosplenic, splenorenal, splenophrenic, and splenocolic ligaments. The splenophrenic and splenocolic ligaments are avascular. The gastrosplenic ligament contains the short gastric vessels. The splenorenal ligament has an anterior and posterior aspect and surrounds the splenic hilum. The splenic hilum contains the splenic artery, splenic vein, and sometimes the tail of the pancreas. The splenic vessels sometimes branch before entering the spleen. The splenic artery is tortuous and is one of the three branches of the celiac trunk. It runs along the superior aspect of the pancreas and gives rise to the short gastric arteries prior to entering the spleen, which course in the gastrosplenic ligament and supply the portion of the greater curvature of the stomach, superior to the splenic artery (Fig. 9.3) (9). The splenic artery also gives rise to the left gastroepiploic artery, which supplies the remainder of the greater curvature of the stomach and the gastrocolic omentum. The splenic vein is slightly inferior and follows the course and branching of the artery.
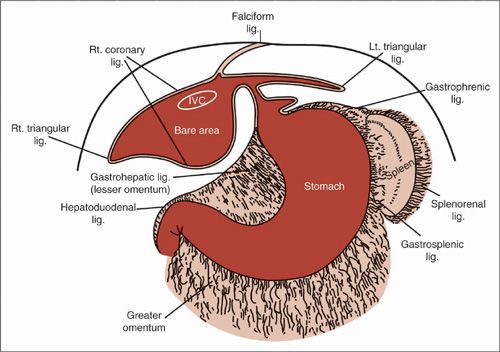
FIGURE 9.2. Peritoneal reflections of the liver: the lesser omentum (hepatogastric and hepatoduodenal ligaments) and its relation to the coronary ligament of the liver and diaphragm.
Source: Reprinted with permission from Skandalakis JE, Gray SW, Rowe JR, eds. Anatomical Complications in General Surgery. New York, NY: McGraw-Hill; 1983.
Patient Positioning
Patient positioning for radical gynecologic oncology procedures is often critical in improving exposure, particularly in obese patients. For most women undergoing radical hysterectomy, ovarian cancer cytoreduction, or pelvic exenterative procedures, we prefer the low lithotomy position using Allen stirrups (Fig. 9.4) (1). The buttocks should extend 2 to 3 cm over the end of the table. This position allows simultaneous access to the perineum and abdomen. The weight of the leg should be on the foot with the legs well padded and attention paid to prevent pressure on the calf and the peroneal nerve. To further improve pelvic exposure, a blanket or pad can be placed under the small of the back.
Abdominal Incisions
Abdominal incisions in gynecologic oncology vary with the indication for the procedure, associated preoperative conditions (such as the presence of ascites or bowel obstruction), suspicion of upper abdominal pathology, and the presence of a previous abdominal scar. Incisions for surgery for gynecologic oncology patients should be highly individualized. Three basic incisions are used for intraperitoneal exposure (Fig. 9.5) (2). Additionally, extraperitoneal access to the pelvic and paraaortic nodes can be achieved through a J-shaped incision (10) or a “sunrise” incision (11).
Transverse incisions offer the advantages of being the best cosmetic incisions for pelvic surgery while also being the least painful, resulting in less interference with postoperative pulmonary function. In addition, compared to vertical incisions, transverse incisions are allegedly stronger and allow better exposure to the pelvic sidewalls. Many gynecologic oncologists use transverse incisions when performing a radical hysterectomy or pelvic exenteration.
In performing the Maylard incision, it is recommended that the deep inferior epigastric vessels be isolated, sectioned, and ligated prior to dividing the rectus muscle (Fig. 9.6) (2). Occasionally, the pelvic surgeon will make a Pfannenstiel incision and find it inadequate. When more exposure is needed, the appropriate maneuver is to convert the Pfannenstiel to a Cherney-type incision. This conversion can be accomplished by dissecting the rectus muscles from the pyramidalis muscles and the anterior rectus sheath, and then transecting the rectus tendons at their insertion into the pubic bone.
Lymph Node Dissection
The surgical technique used to dissect the pelvic and paraaortic lymph nodes involves either a transperitoneal or extraperitoneal approach. The approach utilized is generally dictated by the primary site of disease and the planned accompanying procedure. In cases of endometrial and ovarian carcinoma where the anticipated procedure includes a hysterectomy and/or surgical debulking, the approach is invariably transperitoneal. However, in the pretreatment surgical staging of patients with advanced-stage cervical cancer, the transperitoneal approach has been associated with significant radiation-induced intestinal morbidity due to postoperative adhesion formation (12). Therefore, current clinical trials that require pretreatment surgical staging recommend that the lymph node sampling be performed via the extraperitoneal approach or by operative laparoscopy (13).
FIGURE 9.3. A, B. Variations of splenic vascularization.
Source: Reprinted with permission from Poulin EC, Schlachta DM, Maazza J. Splenectomy. Gastroinestinal tract and abdomen. In: Souba WW, Fink MJ, Jurkovich GJ, et al, eds. ACS Surgery. New York, NY: WebMd; 2005.
FIGURE 9.4. Low lithotomy position using Allen stirrups.
Source: Reprinted with permission from Morrow CP, Curtin JP, eds. Gynecologic Cancer Surgery. New York, NY: Churchill Livingstone; 1996.
Pelvic Lymph Node Dissection
Whether a transperitoneal or extraperitoneal approach is used, the pelvic lymphatic basin can be divided into 5 specific anatomical regions. Most surgeons initially remove pelvic nodes from the external iliac region. Lymphatic-fatty tissue is removed cranially, laterally, and medially from both external iliac vessels and between them. The medial border is formed by the paravesical space. The lateral border is the psoas muscle, the ventral is commonly indicated as the origin of the deep circumflex iliac vein, and the cranial border is the level of the common iliac artery bifurcation, were the lymphatic trunk continues as the superficial common iliac region (Fig. 9.7) (2). The genitofemoral nerve courses laterally to the external iliac artery and should be identified and preserved prior to excising the lymphatic tissue. It usually forms two branches, one running on the surface of the psoas muscle and another through the lymphatic tissue on the external iliac vessels, where it can be easily cut or injured.
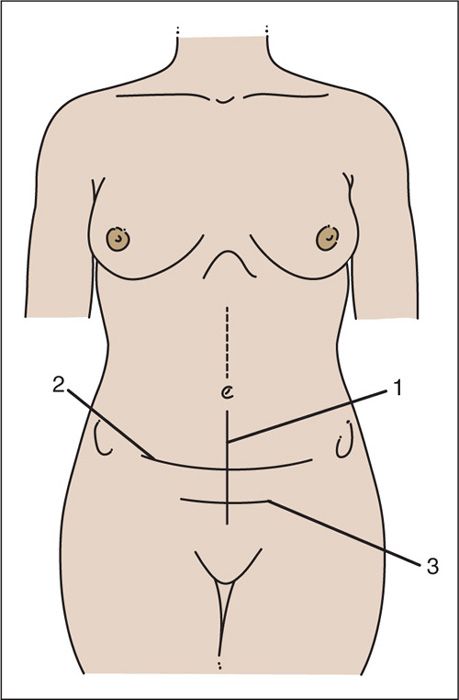
FIGURE 9.5. Entry into the abdominal cavity can be made by three basic incisions: (1) the midline incision; (2) the transverse Maylard-type incision from anterior–superior iliac spine to anterior-superior iliac spine; and (3) the Pfannenstiel incision. The latter is not an incision for radical pelvic surgery, but it can be converted to a Cherney-type incision for improved exposure. For the patient who has some type of transverse incision, and for whom later exposure of the upper abdominal cavity is necessary, a midline upper abdominal incision can be separately used.
Source: Reprinted with permission from Gallup DG, Talledo OE. Surgical Atlas of Gynecologic Oncology. Philadelphia, PA: WB Saunders Co.; 1994:44.
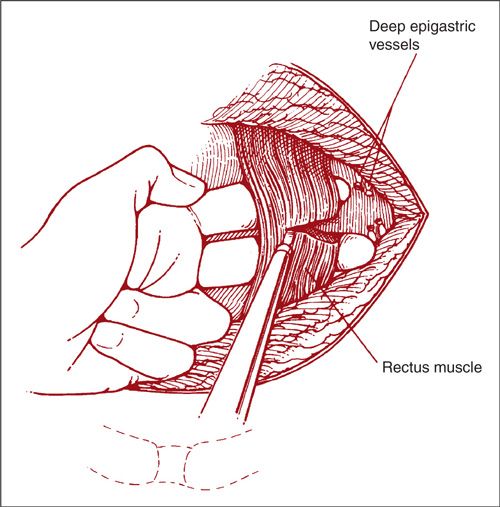
FIGURE 9.6. The Maylard incision. A transverse incision has been made from the anterior-superior iliac spine to the opposite anterior-superior iliac spine. The fascia has been incised transversely. The deep inferior epigastric vessels are located on the lateral and posterior borders of the rectus muscle. They are bluntly dissected from this position by the finger of the operator, isolated, clamped, sectioned, and tied. Only after they are tied should the rectus muscle be incised. This can be done with the Bovie.
Source: Reprinted with permission from Gallup DG, Talledo OE. Surgical Atlas of Gynecologic Oncology. Philadelphia, PA: WB Saunders Co.; 1994:45.
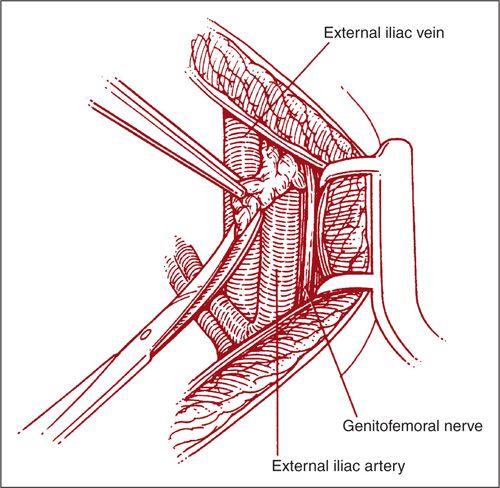
FIGURE 9.7. Starting at the bifurcation of the common iliac vessels, the loose areolar tissue over the vein is excised from cephalad to caudad. Clips should be used at the bifurcation of the common iliac to avoid troublesome bleeding.
Source: Reprinted with permission from Gallup DG, Talledo OE. Surgical Atlas of Gynecologic Oncology. Philadelphia, PA: WB Saunders Co.; 1994:57.
Mobilization and retraction of the external iliac vessels allows access to the obturator region. The cranial border is formed by the common iliac vessels bifurcation, where the lymphatic trunk continues cranially as the deep common iliac region. The medial anatomical border is the paravesical space and the ventral border is formed by the pubic bone together with levator ani and obturator muscles, where the obturator nerve leaves the pelvis through the obturator canal. The lateral border is formed by the obturator internal muscle and the caudal anatomical landmarks are the obturator vessels. The obturator nodes are most easily teased away from the nerve and vessels if one begins the dissection caudally (Fig. 9.8) (2). Usually the last dissected area is the internal iliac region, where the tissue is removed medially from the internal iliac vein.
The dissection continues cranially to the common iliac region. Tissue is removed ventrally and laterally from both common iliac vessels. The lymphatic tissue can be anatomically divided into two parts, the superficial one, which continues mostly from the external iliac region, and the deep one, which runs deeply between the common iliac vein and psoas muscle, continuing from the obturator region. The cranial border is the level of the aorta bifurcation. The genitofemoral nerve should be preserved as it lies on the surface of the psoas muscle. Deeply below the common iliac vessels and the psoas muscle, on the sacral bone, can be identified two nerve structures—the cranial part of the lumbosacral trunk (L4 + L5) (medially) and the obturator nerve (laterally), which enters the region from under the psoas muscle.
If presacral nodes are to be removed, the fatty-lymphatic tissue is removed above the sacral bone, below and between both common iliac veins. The caudal border is indicated by the level of right common iliac vessels bifurcation, where it continues caudally as the internal iliac region. Major branches of the inferior nerve hypogastric plexus should be preserved, as the plexus runs medially on both sides, below the ureters, inside of the mesoureter, which forms a thin layer of tissue between the medial pararectal fossa and large vessels.
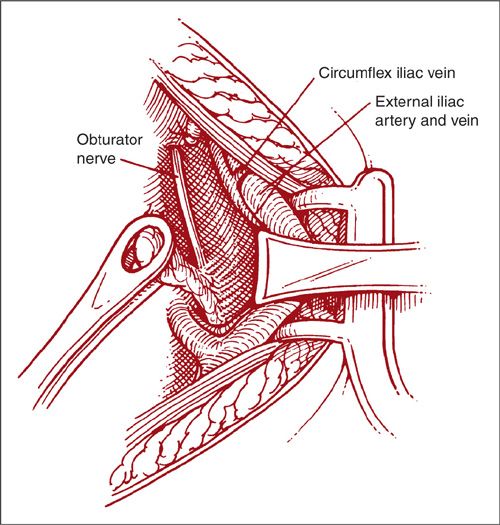
FIGURE 9.8. A vein retractor is used to retract the external iliac veins anterior and lateral to expose the obturator space. Lymphatic tissue is gently teased from the psoas muscle. The entire lymphatic bundle is clamped, sectioned at its caudal end, and ligated at the pelvic sidewall. With the use of the Singley forceps, the lymphatic bundle is bluntly dissected from the obturator nerve and mobilized superiorly. Often, the obturator vein and artery must be sacrificed to obtain access to tissue posterior and lateral to the nerve. Once the tissue is mobilized superiorly, all areolar tissue is cleaned off the hypogastric vessels to the level of the bifurcation of the common iliac artery. The large tissue bundle is clamped and removed en bloc. A tie or clips may be used at the level of the bifurcation.
Source: Reprinted with permission from Gallup DG, Talledo OE. Surgical Atlas of Gynecologic Oncology. Philadelphia, PA: WB Saunders Co.; 1994:58.
Transperitoneal Approach to the Paraaortic Nodes
The transperitoneal approach to the paraaortic lymph nodes can be accomplished by either the direct or the lateral approach. With the direct approach, the dissection begins with an incision in the peritoneum directly over the common iliac arteries and aorta. The lateral approach starts with an incision in the lateral paracolic gutters with subsequent medial reflection of the right and/or left colon. The advantage of the direct approach is that it involves less dissection of the intestine and ureter, whereas one of its main disadvantages is that it is associated with a greater degree of difficulty in exposing the left paraaortic nodes. Consequently, many surgeons sample the right paraaortic (paracaval) lymph nodes via the direct approach and use the lateral approach for the left-sided nodes.
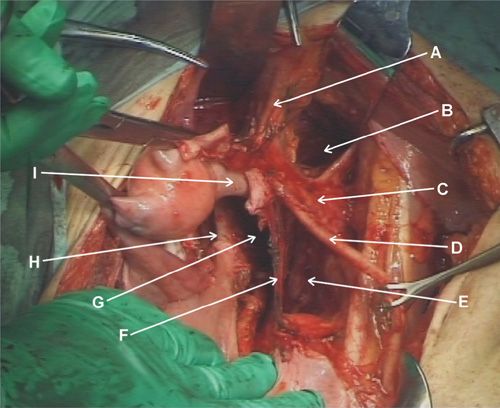
With the direct approach to the right paraaortic nodes, an incision is made in the peritoneum overlying the right common iliac artery (Fig. 9.9). The incision is carried up over the aorta to the level of the duodenum. If the nodal dissection is to be carried out only to the level of the IMA, the duodenum may not need to be mobilized. Using blunt dissection, the ureter and ovarian vessels are identified and mobilized laterally. The lymphatic tissue lateral to the right common iliac artery is elevated and the caudal end is clipped and divided. The dissection then proceeds in a caudad-to-cephalad direction. A plane is created between the IVC and the lymphatic pedicle. The majority of the right paraaortic nodes overlie the IVC and they are generally easily dissected off the vessel. However, there is a fairly constant small vein within the lymphatics anterior to the IVC that inserts just above its bifurcation. If care is not taken to identify and ligate this so-called “fellow’s” vein early in the dissection, it can easily be torn with resultant heavy bleeding (1). When the most cephalad extent of the dissection is reached, the pedicle is clipped or coagulated and divided (Fig. 9.10).
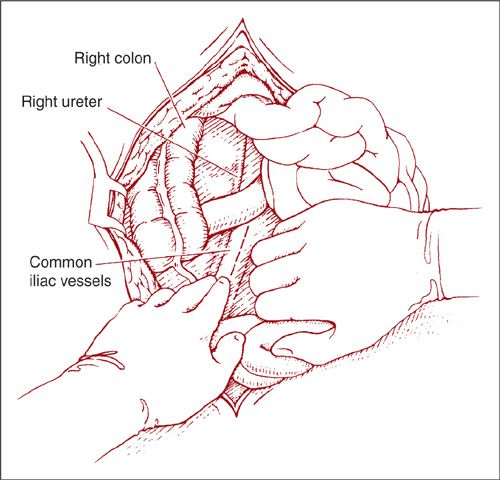
FIGURE 9.9. The small bowel is elevated out of the pelvis placing the mesentery on gentle traction. The right ureter and common iliac artery are identified and the peritoneum overlying the artery is incised.
If the nodes above the IMA need to be sampled, the third portion of the duodenum is mobilized by bilaterally incising the peritoneum around it and then sharply dissecting the areolar tissue underneath (4). The superior portion of the peritoneal incision can be carried up as high as the ligament of Treitz, which can also be divided if needed. Inferiorly, the peritoneal incision is extended over the right ureter around the cecum and up along the right paracolic gutter to mobilize the small bowel mesentery and part of the right colon (Fig. 9.11) (4). The small bowel can then be packed into the upper abdomen or completely removed from the peritoneal cavity and stabilized outside of the abdominal cavity or put into a bowel bag outside the abdomen. The duodenum is retracted superiorly, allowing identification and ligation of the right ovarian artery and vein. The lymphatic tissue can then be safely dissected off of the right side of the aorta and the anterior surface of the IVC up to the level of the renal veins.
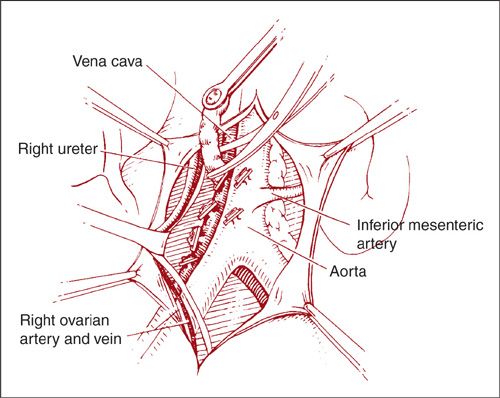
FIGURE 9.10. The specimen is dissected in a cephalad direction. Hemostatic clips are used on either side of the developing pedicle as it is mobilized and divided, and also at the most cephalad extent of the dissection before the specimen is removed.
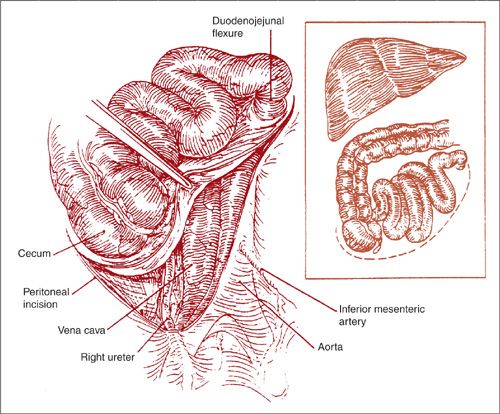
FIGURE 9.11. Extended peritoneal incision. The peritoneal incision is extended over the right ureter around the cecum and cephalad along the right paracolic gutter. This allows for mobilization of the small bowel mesentery as well as the ascending colon.
Source: Reprinted with permission from Fowler JM, Johnson PR. Transperitoneal para-aortic lymphadenectomy. Oper Tech Gynecol Surg 1996;1:9.
The left paraaortic lymph nodes may be removed through the same peritoneal incision. Sharp dissection is used to identify the left common iliac artery, left side of the aorta, IMA, left ureter, and left psoas muscle (Fig. 9.12). The ureter is again mobilized laterally. The lymphatic tissue lateral to the left common iliac artery and aorta is then removed in a caudad-to-cephalad direction. The left paraaortic lymph nodes lie lateral and partially behind the aorta. In dissecting these nodes, judicious use of vascular clips or the use of electrosurgery will help prevent troublesome bleeding from the lumbar vessels. Safe removal of the lymph nodes above the IMA frequently requires identification and division of the left ovarian artery and vein, and occasionally ligation of the inferior mesenteric artery.
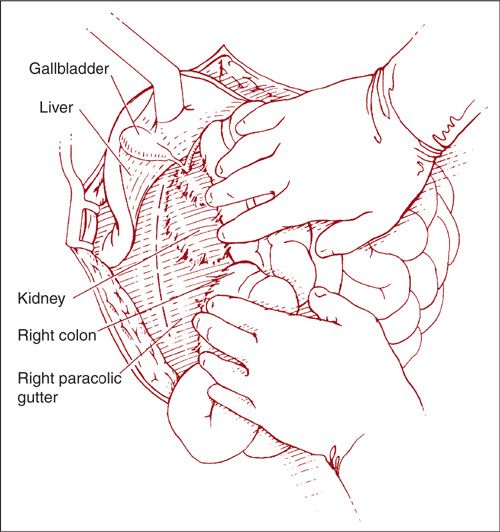
To remove right-sided paraaortic nodes via the lateral approach, the right paracolic gutter is incised along the line of Toldt (Fig. 9.13). The peritoneum is elevated off the psoas muscle and the incision is extended up to the hepatic flexure of the colon. Using sharp and blunt dissection, the right colon is reflected medially. The ureter and ovarian vessels can be identified attached to the undersurface of the reflected peritoneum. They may be left attached or mobilized laterally for better exposure. Further medial mobilization of the colon exposes the IVC and aorta. With the essential structures identified, the lymphatic tissue can then be dissected as previously described in a caudad-to-cephalad direction up to the third portion of the duodenum (Fig. 9.14).
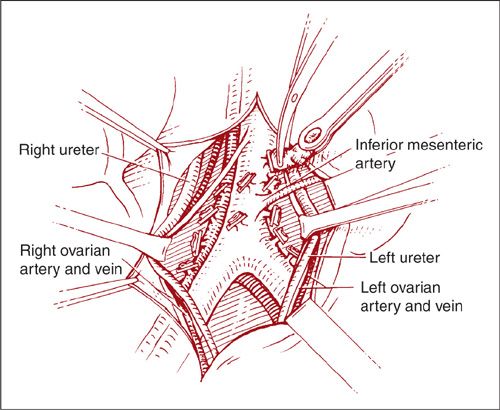
FIGURE 9.12. Removal of the left paraaortic nodes through the same peritoneal incision. The dissection also proceeds in a cephalad direction, again using hemostatic clips on the lateral and medial margins. Care should be taken to avoid injury to the inferior mesenteric artery that arises approximately 3 to 4 cm above the aortic bifurcation.
FIGURE 9.13. The right paracolic gutter is exposed by medial traction on the ascending colon. The gutter is incised along the line of Toldt.
If the lymph nodes above the IMA need to be sampled, the Kocher maneuver is used to reflect the duodenum medially. The peritoneum lateral to the convexity of the C-curve of the duodenum is incised and the second portion of the duodenum is then dissected off of the IVC. For further exposure, the peritoneal incision along the line of Toldt may need to be extended cephalad to mobilize completely the hepatic flexure of the colon (Fig. 9.15). The right ovarian artery and vein are identified and divided. The right-sided paraaortic lymph nodes are then able to be dissected off of the IVC and right aorta up to the level of the renal vessels.
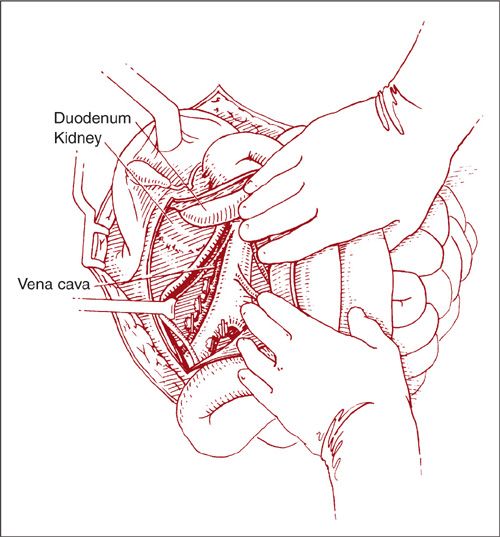
FIGURE 9.14. With the ureter and ovarian vessels identified, the dissection begins at the right common iliac artery and proceeds cephalad up to the third portion of the duodenum.
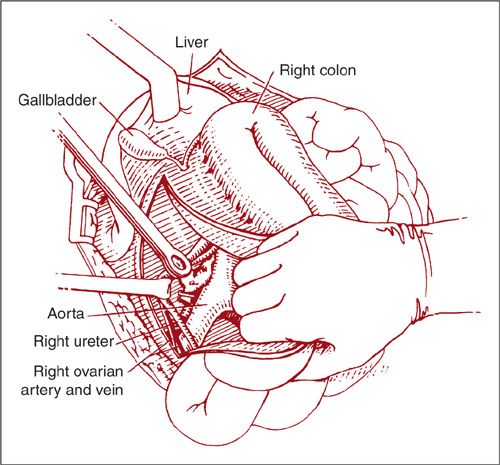
FIGURE 9.15. The Kocher maneuver can be used to gain access to the lymph nodes above the IMA. The peritoneum lateral to the convexity of the C-curve of the duodenum is incised and the second portion of the duodenum is then dissected off of the IVC. The common bile duct and pancreatic duct enter the second portion of the duodenum posteromedially. For further exposure, the incision along the line of Toldt can be extended cephalad to mobilize completely the hepatic flexure of the colon.
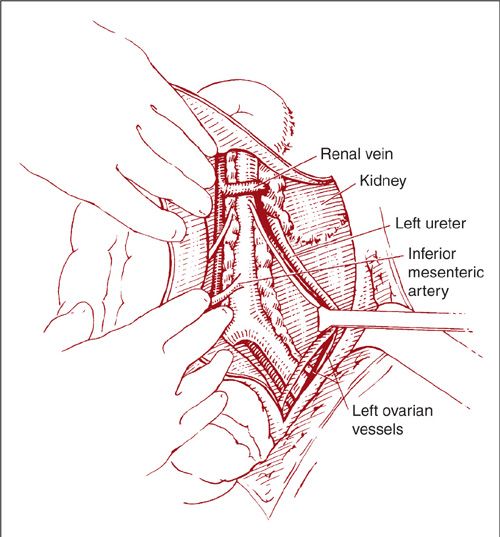
The lateral approach to the left paraaortic lymph nodes is accomplished in a similar fashion by incising along the line of Toldt and mobilization of the left colon medially (Fig. 9.16). Again, the ureter and ovarian vessels are identified on the undersurface of the reflected peritoneum, and they may be left attached or mobilized laterally for better exposure (Fig. 9.17). After further mobilization of the left colon and identification of the aorta and the IMA, the left-sided nodes are removed in a caudad-to-cephalad direction (Fig. 9.18). Dissection of the nodes above the IMA requires mobilization of the splenic flexure of the colon, division of the left ovarian artery and vein, and occasionally ligation of the inferior mesenteric artery.
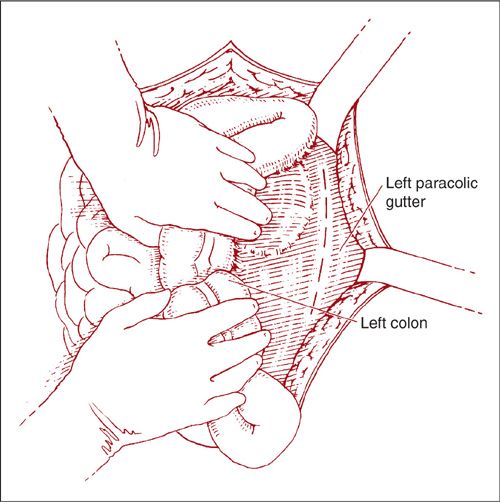
FIGURE 9.16. The lateral approach to the left paraaortic nodes is accomplished by retracting the descending colon medially and incising along the line of Toldt.
FIGURE 9.17. Using sharp and blunt dissection, the left colon can be mobilized medially, exposing the left ureter, ovarian vessels, and aorta.
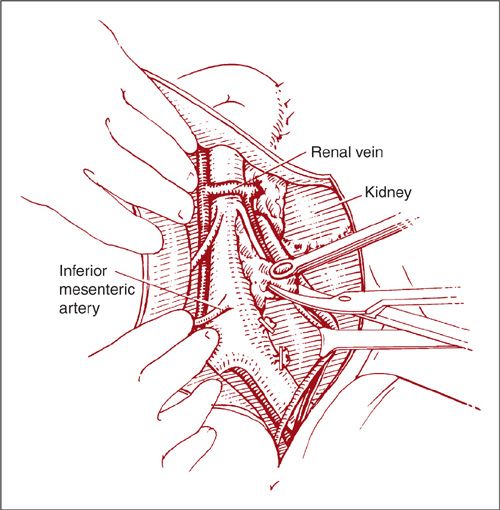
FIGURE 9.18. Dissection begins at the left common iliac artery and proceeds cephalad using hemostatic clips. Care should be taken to avoid injury to the inferior mesenteric artery that arises approximately 3 to 4 cm above the aortic bifurcation.
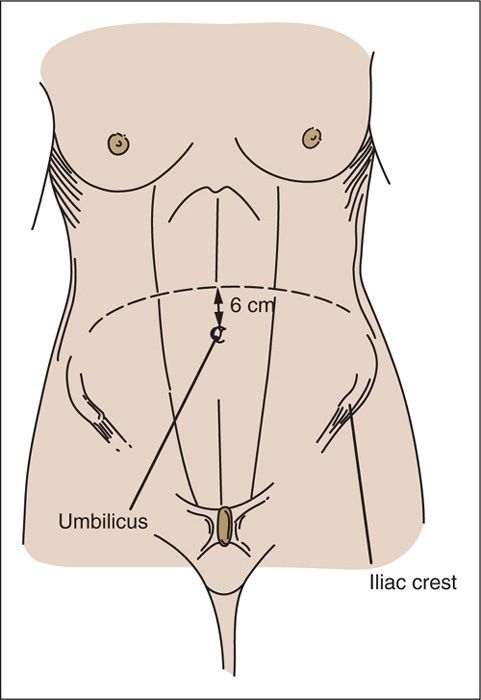
FIGURE 9.19. The “sunrise” incision. In the center, the incision is approximately 6 cm above the umbilicus. The incision is carried laterally in a downward fashion to the level of the iliac crests.
Source: Reprinted with permission from Gallup DG, Talledo OE. Surgical Atlas of Gynecologic Oncology. Philadelphia, PA: WB Saunders Co.; 1994:118.
Extraperitoneal Approach to the Paraaortic Nodes
The extraperitoneal approach to the paraaortic lymph nodes by means of a supraumbilical transverse sunrise incision was initially described by Gallup et al. (11). The skin incision is made 6 cm above the umbilicus in the midline and is carried laterally and caudad to the level of the iliac crests bilaterally (Fig. 9.19) (2).
The fascia is incised transversely. The rectus muscles are dissected off of the anterior-lying fascia cephalad and caudad. The right rectus muscle is transected. The right transversus muscle is then identified and transected caudally and laterally. The hand of the operator is inserted deep into the incision until the right psoas muscle and external iliac vessels are palpated. The peritoneum is then bluntly dissected from caudad and lateral to cephalad and medial, separating it from the underlying common iliac vessels until the great vessels are exposed (Fig. 9.20) (2). If the peritoneum is inadvertently entered, it must be closed immediately.
After identification of the right ureter and ovarian vessels, the right paraaortic nodes can be removed. In thin patients, the left paraaortic nodes may be able to be removed through a right abdominal approach. However, if exposure is difficult, the left rectus and transversus muscles can be transected and the peritoneum mobilized medially in a similar fashion to gain access to the left paraaortic nodes.
Radical Hysterectomy
Although radical hysterectomy is a traditional procedure in gynecologic oncology, with a history of almost 100 years, its performance is still poorly standardized. Different terminology and classifications are currently used to describe individual types of the procedure. As a consequence, the extent of the parametria resection may vary substantially between institutions and surgeons, even if the same terminology is used.
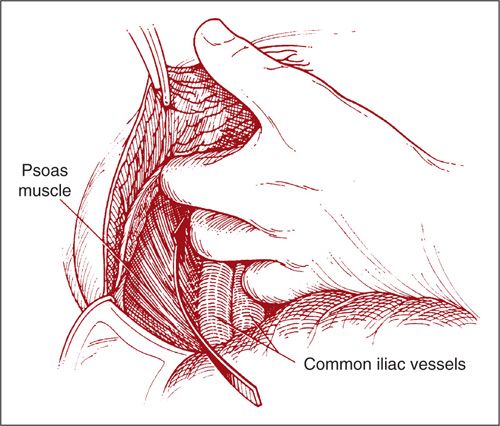
FIGURE 9.20. With the rectus and transversus muscles transected, the operator’s hand is inserted caudad until the psoas muscle and external iliac vessels are palpated. The peritoneum is then bluntly dissected from caudad and lateral to cephalad and medial, separating it from the underlying common iliac vessels until the aorta and vena cava are exposed.
Source: Reprinted with permission from Gallup DG, Talledo OE. Surgical Atlas of Gynecologic Oncology. Philadelphia, PA: WB Saunders Co.; 1994:121.
There are several key principles for the classification of radical hysterectomies. First, the key and sole parameter for differentiation between types of radical hysterectomy should be the extent of parametria resection. The resection or removal of other organs or structures should not determine the type of the procedure, including the size of vagina resection. Second, the extent of resection should be precisely defined for all three parts of the parametria (ventral, lateral, dorsal) and in three planes (sagittal, frontal, and transverse) (Fig. 9.21). Mainly surgical margins in vertical (deep parametrial) dimension determine long-term morbidity due to the localization of vegetative nerves in the caudal part of all 3 parts of the parametria. Third, the extent of the procedure and its classification may be different on each side of the cervix if the tumor growth is asymmetrical. Fourth, although the classification system is usually proposed for radical hysterectomy, it is applicable to the radical trachelectomy and the radical parametrectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree