Background
Surgery is currently the mainstay treatment for solid tumors and many benign diseases, including endometriosis, and women tend to receive substantially more surgeries than men mainly because of gynecological and cosmetic surgeries. Despite its cosmetic, therapeutic, or even life-saving benefits, surgery is reported to increase the cancer risk and promotes cancer metastasis. Surgery activates adrenergic signaling, which in turn suppresses cell-mediated immunity and promotes angiogenesis and metastasis. Because immunity, angiogenesis, and invasiveness are all involved in the pathophysiology of endometriosis, it is unclear whether surgery may accelerate the development of endometriosis.
Objective
The objective of the study was to test the hypothesis that surgery activates adrenergic signaling, increases angiogenesis, and accelerates the growth of endometriotic lesions.
Study Design
This was a prospective, randomized experimentation. The first experiment used 42 female adult Balb/C mice, and the second used 90 female adult Balb/C mice. In experiment 1, 3 days after the induction of endometriosis, mice were randomly divided into 3 groups of approximately equal sizes, control, laparotomy, and mastectomy. In experiment 2, propranolol infusion via Alzet pumps was used to forestall the effect of sympathetic nervous system activation by surgery. In both experiments, mice were evaluated 2 weeks after surgery. Lesion size, hotplate latency, and immunohistochemistry analysis of vascular endothelial growth factor, CD31-positive microvessels, proliferating cell nuclear antigen, phosphorylated cyclic adenosine monophosphate-responsive element-binding protein, β 2 -adrenergic receptor (ADRB)-2, ADRB1, ADRB3, ADRA1, and ADRA2 in ectopic implants.
Results
Both mastectomy and laparotomy increased lesion weight and exacerbated hyperalgesia, increased microvessel density and elevated the immunoreactivity against ADRB2, phosphorylated cyclic adenosine monophosphate–responsive element-binding protein, vascular endothelial growth factor, and proliferating cell nuclear antigen but not ADRB1, ADRB3, ADRA1, and ADRA2, suggesting activated adrenergic signaling, increased angiogenesis, and accelerated growth of endometriotic lesions. β-Blockade completely abrogated the facilitory effect of surgery, further underscoring the critical role of β-adrenergic signaling in mediating the effect of surgery.
Conclusion
Surgery activates adrenergic signaling, increases angiogenesis, and accelerates the growth of endometriotic lesions in the mouse, but such a facilitory effect of surgery can be completely abrogated by β-blockade. Whether surgery can promote the development of endometriosis in humans warrants further investigation.
Surgery is now the mainstay treatment for solid tumors and many benign diseases, including endometriosis, defined as the presence of endometrial-like tissues outside the uterine cavity. Remarkably, women tend to receive substantially more surgeries than men mainly because of gynecological surgeries.
In the United States, more than 90% of cosmetic and reconstructive surgeries are performed on women. Conceivably, such a predominance of women in plastic surgeries is similar elsewhere in the world.
Despite its cosmetic, therapeutic, or even life-saving benefits, surgery also has its down-side. Extensive animal studies and considerable human studies suggest that surgical stress promotes cancer metastasis.
Noncancer-related surgery may also increase the risk of development of cancer. Hysterectomy, for example, is reported to increase the risk of renal cancer, and total joint arthroplasty is said to elevate the risk of prostate cancer. Surgical stress is shown to delay prostate involution in mouse. Even 1 single core needle biopsy is reported to promote the distant metastasis of breast cancer in mouse.
Surgery inevitably results in tissue damage, a trauma, or stress to the body. As such, various bioactive molecules are secreted perioperatively, including catecholamines that are known to suppress cell-mediated immunity and promote angiogenesis and metastasis in animal studies.
Currently the pathogenesis of endometriosis, a common disorder among women of reproductive age and a major contributor to pelvic pain and infertility, is poorly understood. Consequently, its effective treatment is still a challenge. The quest for novel nonhormonal therapeutics so far has not been successful, and there is no single biomarker that has been unequivocally shown to be clinically useful in diagnosing endometriosis.
Because immunity, angiogenesis, and invasiveness are all involved in the pathophysiology of endometriosis, it is biologically plausible that surgery may also exert its promotional effect on endometriosis. However, to the best of our knowledge, so far there has been no published report on the effect of surgery on the development of endometriosis or on the high recurrence risk of endometriosis. Therefore, we conducted this study and evaluated the effects of surgery, if any, on endometriotic lesion growth and angiogenesis in a mouse model of endometriosis.
We evaluated the effects of 2 modes of surgery, one mimicking laparotomy and the other, mastectomy. Conceivably, mastectomy is more localized and less invasive or traumatic than laparotomy and may have a less direct effect on lesion growth. Representing different stress levels, these 2 modes should help us to assess the effect of surgery, if any, on the development of endometriosis more reliably.
In addition, given the finding that the β 2 adrenergic receptor appears to be involved in surgery-facilitated endometriosis development, we evaluated whether β-blockade could forestall the promotional effect induced by surgery.
Materials and Methods
Animals
A total of 132 7 week old virgin female Balb/C mice were purchased from the SLAC Experimental Animal Company (Shanghai, China) and used for this study. All mice were maintained under controlled conditions with a 12 hours light/12 hours dark cycle and had access to food and water ad libitum.
All experiments were performed under the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Experimental Animals Review Board of Shanghai Obstetrics and Gynecology Hospital, Fudan University.
Induction of endometriosis
We used an established mouse model of endometriosis by intraperitoneal injection of endometrial fragments as described and also used in our previous studies. Briefly, 7 week old donor mice were initially injected with 100 μg/kg estradiol benzoate (Animal Medicine Factory, Hangzhou, China). One week later they were killed, and their uteri were removed and harvested. The uterine tissues were seeded in a Petri dish containing warm sterile saline and split longitudinally with a pair of scissors.
Two uterine horns from each mouse were first minced with scissors, ensuring that the maximal diameter of the fragment was consistently smaller than 1 mm. Then the fragments were injected intraperitoneally to recipient mice. Two recipient mice received the fragment preparation derived from 1 donor mouse. To eliminate any potential bias, endometrial fragments from 3 donor mice were mixed together and injected intraperitoneally to 6 mice, 2 each from 1 of the 3 groups for experiment 1 or 1 each from the 6 groups for experiment 2.
Models of surgical stress
Mice were anaesthetized with 300 mg/kg chloral hydrate and exposed to an experimental mastectomy or laparotomy as described previously. Mastectomy was performed by the midline chest wall skin incision and removal of 1 of the right mammary tissues from the chest wall, followed by the closure of the skin of chest wall with 3–4 surgical clips.
The laparotomy was done by a 3 cm midline abdominal incision followed by the externalization of intestines for a period of 4 minutes ; at the same time, the small intestine was rubbed with 2 saline-soaked cotton swabs in 4 different locations to simulate a surgical procedure. The intestine was then returned to the abdominal cavity and irrigated with saline, and the abdominal wall was closed with surgical clips. After surgery, all mice were administrated with penicillin of 40,000 U intramuscularly to prevent infection.
Mouse experiment 1: surgery accelerates the growth of endometriotic lesions
After 1 week of acclimation, 28 mice were randomly divided into 3 groups: the control group (n = 9 ), the mastectomy group (n = 10), and the laparotomy group (n = 9). Before induction of endometriosis, the baseline body weight and hotplate latency were measured and recorded. Three days after the induction, simulated mastectomy and laparotomy were performed (see Models of surgical stress discussed in the text previously).
To rule out any possible effect of anesthesia on endometriosis development, mice in the control group did not receive any surgery but were anaesthetized in the same manner as the other 2 groups. Fourteen days after surgery, body weight and hotplate latency were measured before mice were killed by cervical dislocation. The abdominal cavities were immediately opened up and all lesions were excised and processed for quantification and immunohistochemistry analysis. The extent of the induced endometriosis was evaluated by assessing the weight of all lesions from each mouse.
The hotplate test was performed with a commercially available Hot Plate Analgesia Meter (model BME-480; Institute of Biomedical Engineering, Chinese Academy of Medical Sciences, Tianjin, China) as reported previously and is described in more detail in the Supplementary Appendix .
Mouse experiment 2: intervention by β-blockade
To further confirm the role of β-adrenergic signaling in surgery-accelerated lesion growth and to evaluate the effect of β-blockade, we used propranolol hydrochloride (5 mg/kg per day; Sigma, St Louis, MO), a nonspecific β-adrenergic receptor (ADRB) antagonist, given via Alzet miniosmotic pumps (model 1002; DURECT Corp, Cupertino, CA), which ensured consistent and controlled release of contents for 14 days. For controls, Alzet pumps containing only phosphate-buffered saline (PBS) of the same amount as propranolol was used. The minipumps containing PBS or propranolol were inserted on the nape of the neck 7 days before surgery (ie, 3 days before the induction of endometriosis).
After 3 days of acclimation, 60 mice were randomly divided into 6 groups of equal sizes, which received the following treatments: control (no surgery) plus PBS, mastectomy plus PBS, laparotomy plus PBS, control plus propranolol (no surgery), mastectomy plus propranolol, and laparotomy plus propranolol.
Before the insertion of the pumps and the induction of endometriosis, body weight and hotplate latency were measured and recorded. Surgery was performed 3 days after the induction of endometriosis (see Models of surgical stress discussed in previous text). Mice in the control group were only anesthetized similarly to those receiving simulated surgery.
Two weeks after surgery, body weight and hotplate latency were measured and recorded before the animals were killed by cervical dislocation. The abdominal cavities were immediately opened up and all lesions were excised and processed for quantification and immunohistochemistry analysis.
Immunohistochemistry
Tissue samples were fixed with 10% formalin (wt/vol) and paraffin embedded. Serial 4 μm sections were obtained from each block, with the first resultant slide being stained for hematoxylin and eosin to confirm pathological diagnosis, and the subsequent slides stained for vascular endothelial growth factor (VEGF), CD31-positive microvessels, proliferating cell nuclear antigen (PCNA), phosphorylated cyclic adenosine monophosphate (cAMP)–responsive element-binding protein (p-CREB), ADRB2, ADRB1, ADRB3, α 1 -adrenergic receptor (ADRA1), and ADRA2.
Routine deparaffinization and rehydration procedures were performed. For antigen retrieval, the slides were heated at 98°C in an EDTA buffer (pH 8.0; Shanghai Sun BioTech Co, Shanghai, China) for a total of 20 minutes for staining for CD31, ADRB2, p-CREB, ADRA1, and ADRA2, or citrate buffer (pH6.0) for a total of 30 minutes for staining for PCNA, VEGF, ADRB1, and ADRB3 and then cooled naturally to the room temperature. The Table lists the names, along with catalog numbers, of the primary antibodies used in this study and their respective diluted concentrations.
| Antibody name | Catalog number | Concentration |
|---|---|---|
| PCNA | Ab29 | 1:50 |
| VEGF | Ab52917 | 1:500 |
| CD31 | Ab28364 | 1:50 |
| ADRB2 | Ab61778 | 1:50 |
| p-CREB | Ab32096 | 1:100 |
| ADRB1 | Ab3442 | 1:400 |
| ADRB3 | Ab94506 | 1:100 |
| ADRA1 | Ab3462 | 1:100 |
| ADRA2 | 70R-13686 | 1:200 |
All sections were incubated with the primary antibody overnight at 4°C. After slides were rinsed, the horseradish peroxidase–labeled secondary antibody detection reagent (Sunpoly-HII; BioSun Technology Co, Ltd, Shanghai, China) was incubated at room temperature for 30 minutes. The bound antibody complexes were stained for 3-5 minutes or until appropriate for microscopic examination with diaminobenzidine and then counterstained with hematoxylin (30 seconds) and mounted.
Images were obtained with a microscope (Olympus BX51; Olympus, Tokyo, Japan) fitted with a digital camera (Olympus DP70; Olympus). The microvessel density was evaluated, by the same investigator without knowledge of the group identify of the mouse being evaluated, by counting CD31-labeled blood vessels in 5 chosen ×400 Hp field and then averaged, as reported previously.
For other immunostained markers, the quantification was made through 3-5 randomly selected images for each mouse at ×400 magnification taken to obtain a mean optional density value by Image Pro-Plus 6.0 (Media Cybernetics, Inc, Bethesda, MD), as reported previously.
Human brain, mouse liver tissue, or human invasive thyroid cancer samples were used as positive controls for ADRB2, ADRB1, ADRB3, ADRA1, ADRA2, CD31, PCNA, VEGF, and p-CREB. For negative controls, mouse ectopic endometrial tissue samples were incubated with rabbit or mouse serum instead of primary antibodies.
ADRB2 and VEGF immunoreactivity was seen primarily in glandular epithelial cells and was localized in the cytoplasm. PCNA and p-CREB immunoreactivity was seen both in glandular epithelial cells and stromal cells and was localized in the cell nucleus. However, only the epithelial component of PCNA and p-CREB staining was used for analysis because of their predominance in that compartment.
CD31 immunostaining was seen mostly in vascular endothelial cells and stained positive exclusively in the epithelium of blood vessels. The microvessel density was calculated and averaged over 5 randomly selected areas of vessels (except large ones) in ectopic endometrium. To minimize potential bias, the person who evaluated the slides was blinded as to slide group.
Statistical analysis
The comparison of distributions of continuous variables between or among 2 or more groups was made using the Wilcoxon and Kruskal test, respectively, and the paired Wilcoxon test was used when the before-after (induction of endometriosis) comparison was made for the same group of subjects.
The Pearson’s or Spearman’s rank correlation coefficient was used when evaluating correlations between 2 variables when both variables were continuous or when at least 1 variable was ordinal. Multivariate linear regression analyses were used to determine which factors were associated with lesion weight, hotplate latency, or immunostaining levels. Dummy variables were used to indicate whether the mouse received a mastectomy or not, laparotomy or not, and β-blocker treatment or not.
Values of P < .05 were considered statistically significant. All computations were made with R 3.2.2 ( www.r-project.org ).
Results
Surgery accelerates the development of endometriosis
To mimic the effect of surgery, we performed laparotomy on mice 3 days after the induction of endometriosis. We performed a mastectomy on an additional group of mice to account for possible local effects. We found no difference in body weight among the 3 groups of mice before the induction of endometriosis and by the time when mice were killed (both values of P > .70). However, both laparotomy and mastectomy resulted in significantly increased lesion weight ( P = .014 and P = .035, respectively; Wilcoxon test; Figure 1 A ), on average by 85.6% and 67.2%, respectively.
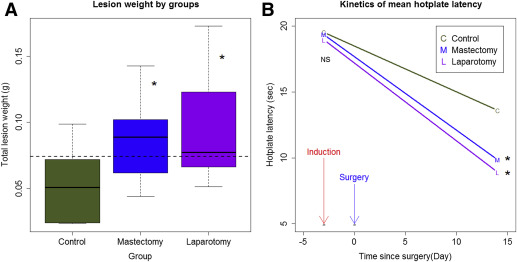
Multivariate linear regression analysis indicated that both laparotomy and mastectomy, but not body weight, were positively associated with the lesion weight ( P = .0040 and P = .0098, respectively; R 2 = 0.32; lesion weight was log transformed to improve normality).
Consistent with the increased lesion weight in mice that had undergone laparotomy and mastectomy, the hotplate latencies in mice that had undergone laparotomy and mastectomy were significantly shorter than mice without surgery ( P = .031 and P = .043, respectively; Figure 1 B), even though all groups of mice had significantly reduced latency as compared with the baseline level ( P = 7.5 × 10 −9 ; Figure 1 B).
Multivariate linear regression analysis of the change in latency indicated that both laparotomy and mastectomy resulted in a further decrease in hotplate latency as compared with the control group ( P = .009 and P = .027, respectively; R 2 = .60).
Effect of surgery on expression of adrenergic receptors and angiogenesis and proliferation markers
We next examined immunoreactivity to CD31 (a marker for microvessel density), ADRB2, VEGF, PCNA, p-CREB, ADRA1, ADRA2, ADRB1, and ADRB3 in ectopic lesions. As shown in Supplemental Figure 1 (Supplemental Information), ADRB2 and VEGF immunoreactivity was seen primarily in glandular epithelial cells and was localized in the cytoplasm. PCNA and p-CREB immunoreactivity was seen both in glandular epithelial cells and stromal cells and were localized in the cell nucleus, but the change of immunoreactivity in glandular epithelial cells was more prominent. CD31 immunostainings were seen mostly in vascular endothelial cells.
We found that both laparotomy and mastectomy resulted in higher immunoreactivity against ADRB2 in ectopic endometrium as compared with control mice ( P = 4.9 × 10 −5 and P = .0015, respectively, by multivariate linear regression analysis; R 2 = 0.50; Supplemental Figure 1 and Figure 2 A ).
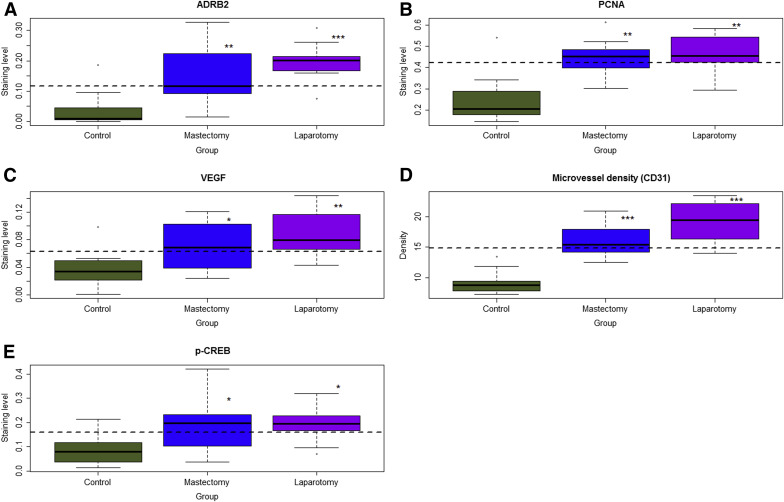
Consistent with increased lesion weight, both laparotomy and mastectomy yielded higher immunostaining levels of PCNA and VEGF and increased microvessel density as compared with controls (all values of P < .024 by linear regression analyses, all R 2 > 0.34; Supplemental Figure 1 and Figure 2 , B–D).
In addition, both laparotomy and mastectomy resulted in higher immunoreactivity against p-CREB ( P = .014 and P = .028, respectively; R 2 = 0.25; Supplemental Figure 1 and Figure 2 E). However, no difference in immunoreactivity against ADRB1, ADRB3, ADRA1, and ADRA2 was found among the 3 groups of mice (all values of P > .75; Supplemental Figure 2 in Supplemental Information).
The microvessel density correlated positively with VEGF staining (r = 0.53, P = .0034), PCNA (r = 0.69, P = 5.7 × 10 −5 ), and ADRB2 (r = 0.57, P = .0016). ADRB2 staining levels correlated positively with PCNA (r = 0.49, P = .008) and p-CREB (r = 0.59, P = 9.5 × 10 −4 ).
β-Blocker abrogates surgery-accelerated lesion growth
Given the role of ADRB2 in surgery-accelerated lesion growth, we then conducted a second experiment using a nonspecific β-blocker. Our goal was 2-fold: to confirm the role of ADRB2 in facilitating endometriosis development induced by surgery and to see whether β-blockade can abrogate this acceleration.
As expected, there was no difference in body weight measured 4 days before endometriosis induction (just before the insertion of the minipump) and just prior to the induction among the 6 groups of mice ( P = .89 and P = .24, respectively). However, 2 weeks after surgery, there was a significant difference in body weight among the groups ( P = .010; Supplemental Figure 3 , Supplemental Information).
A multivariate linear regression analysis with previously measured body weight, mastectomy or not, laparotomy or not, and the use of β-blocker or not as covariables indicated that the use of a β-blocker was associated with a decrease in body weight ( P = .0032), whereas the body weight measured previously (3 days before surgery) was associated with the increase in body weight ( P = .0003; R 2 = 0.39).
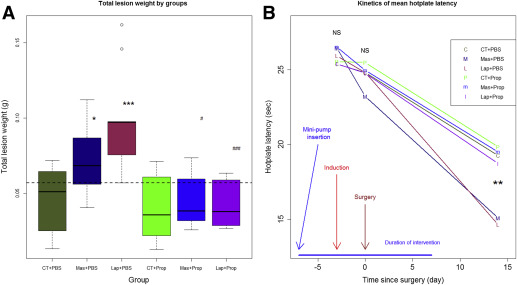
Consistent with the results from experiment 1, both laparotomy and mastectomy increased lesion weight by an average of 119.6% and 64.9%, respectively, as compared with the control group ( P = .0010 and P = .043, respectively; Figure 3 A ). However, treatment with a continuous infusion of propranolol completely abrogated the increased lesion growth ( P = .00029 and P = .013, respectively, for laparotomy and mastectomy groups; Figure 3 A).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


