Christopher K. McQuitty, MD
Erin G. Sreshta, MD
RISK OF HEART DISEASE IN PREGNANCY
HEMODYNAMIC AND HEMOSTATIC CHANGES IN PREGNANCY
MANAGEMENT AND MODE OF DELIVERY
• Prosthetic Valves and Anticoagulation
3. Low-Molecular-Weight Heparin
4. Recommendations for Pregnant Patients with Mechanical Prosthetic Valves
6. Management of Aortic Disease
• Acyanotic Lesions With Shunt
• Acyanotic Lesions Without Shunt
12. dextro—Transposition of the Great Arteries
CARDIAC SURGERY DURING PREGNANCY
INTRODUCTION
Cardiac disease complicates only 1% to 4% of pregnancies in the United States, yet it is still the most important cause of nonobstetric maternal morbidity and mortality.1 Structural heart disease refers to an interruption of the natural flow of blood through the chambers and valves of the heart. The pathology may be congenital or acquired, typically involving myocardial and valvular lesions. Structural heart disease also includes noncoronary cardiovascular processes and the related interventional procedures to repair the defects.2 Ischemic disease (atherosclerosis of native coronary arteries) and cardiomyopathies presenting with pulmonary edema are discussed separately (see Chapters 6 and 8).
Preconception counseling and planned pregnancy after risk stratification of every woman with structural heart disease in the child-bearing period is important to reduce the maternal and fetal morbidity and mortality.3 Successful pregnancies can be achieved when cardiac complications are managed during pregnancy, using a multidisciplinary team approach for diagnosis and treatment. The team should have a cardiologist, obstetrician, and anesthesiologist with experience in cardiac disease in pregnancy. Higher risk cases may require the immediate availability of a neonatal specialist and a cardiothoracic surgeon. Antenatal care should focus on optimization of cardiovascular function with appropriate dosing of medications, some of which may need to be altered to avoid teratogenicity. The plan for management of labor, delivery, and the postpartum should be in place before the third trimester.
Diagnosis
If there is a strong suspicion of structural heart disease during pregnancy, confirmatory tests should be initiated. Echocardiography should be performed in any pregnant patient with unexplained or new cardiovascular signs or symptoms.4 When ventricular function cannot be accurately assessed by echocardiography, magnetic resonance imaging (MRI) should be performed. MRI may be used to evaluate complex heart disease or aortic pathology. Chest radiography and computed tomography (CT), with ionizing radiation risks, are usually not necessary to diagnose structural heart disease. One exception for the use of CT during pregnancy is to diagnose or exclude pulmonary embolism.4
RISK OF HEART DISEASE IN PREGNANCY
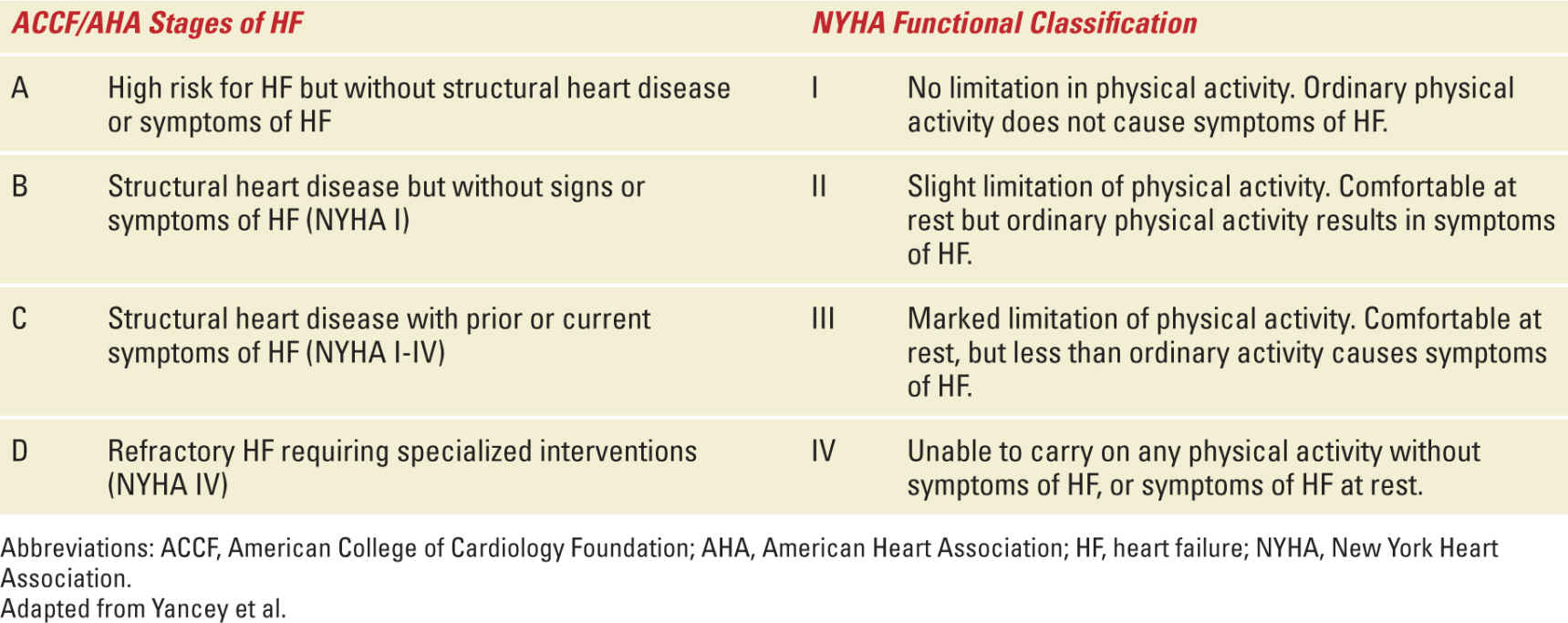
Several studies have shown that women with heart disease have an increased risk of maternal and neonatal complications. A registry with patients from 28 countries concluded that most women with structural heart disease have safe pregnancies as long as pre-pregnancy counseling and specialized care are available. Maternal functional status before pregnancy is one of the most important predictors of both maternal and neonatal outcomes. Pregnancy outcome is better in New York Heart Association (NYHA) class I/II women compared with class III/IV and in those with successful cardiac interventions before pregnancy5 (see Table 5-1). Despite high-quality care, these patients must recognize that complication rates are higher and maternal mortality may approach 1% (compared with 0.007% in the normal population).6
Classification of Heart Failure |
An analysis of high-risk parturients in a tertiary center showed that the (Cardiac Disease in Pregnancy) CARPREG risk index may be used to predict complications in pregnant women with heart disease.7 The CARPREG study analyzed 562 pregnant women with heart disease and identified 4 predictors for maternal complications. There was a 75% risk of a cardiac event if patients had >1 of these factors8:
• A prior cardiac event such as heart failure, transient ischemic attack or stroke before pregnancy, or arrhythmias
• A pre-pregnancy NYHA class greater than II or the presence of cyanosis
• Left heart obstruction with a mitral valve area less than 2 cm2, or aortic valve area less than 1.5 cm2, or an aortic outflow gradient >30 mmHg
• A left ventricular ejection fraction (LVEF) less than 40%.
A cardiac event was defined as pulmonary edema, arrhythmia, cerebrovascular event, cardiac arrest, or death. There is also an increased risk of neonatal complications in women with heart disease; these maternal predictors include NYHA > II or cyanosis, maternal left heart obstruction, smoking during pregnancy, multiple gestation, oral anticoagulation during pregnancy, and mechanical valve prosthesis.4
Pregnancy has classically been contraindicated and termination of pregnancy considered in the following conditions3:
• Severe pulmonary hypertension (pulmonary artery [PA] systolic pressure >75% of systemic systolic pressure) (see also Chapter 11)
• Dilated cardiomyopathy with congestive heart failure
• Cyanotic congenital heart disease (CHD)
• Severe symptomatic stenotic valve disease, which cannot be treated using percutaneous procedures
• Severe mitral regurgitation (MR) and aortic regurgitation (AR) with left ventricular (LV) dysfunction (LVEF <40%).
The maternal risk classification of the World Health Organization (WHO) integrates all maternal risk factors and includes contraindications not included in the CARPREG study.4 Risk stratification by the WHO classification (see Table 5-2) has been shown to be a valid model to predict pregnancy outcome in patients with structural heart disease.9 Women in WHO class I or II need minimal intervention and should be seen by cardiology at least every trimester. Those in WHO class III have a higher risk of complications and should be evaluated by cardiologist and obstetricians at least monthly. WHO class IV patients should avoid pregnancy; if pregnancy occurs and termination is not an option, they should be evaluated in a tertiary care center bimonthly and hospitalized if symptoms occur.
Modified WHO Classification of Maternal Risk for Structural Heart Lesions |
Abbreviations: LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Adapted with permission from Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC), Eur Heart J. 2011;32(24):3147-3197.
HEMODYNAMIC AND HEMOSTATIC CHANGES IN PREGNANCY
Women with structural heart disease may have an attenuated ability to adapt to the cardiovascular changes associated with pregnancy. These patients may have a reduction in systolic function and a progression of diastolic dysfunction that may persist up to 6 months after pregnancy.10 Although mild dyspnea with activity may occur in normal pregnancies, worsening of dyspnea with gestational age or occurring at rest should be evaluated. Angina, syncope, and most diastolic murmurs mandate further assessment in the hospital. A normal echocardiographic exam of a parturient would reveal mild ventricular chamber enlargement, which can cause mild MR. Mild pulmonic and tricuspid regurgitation is also common.
The adaptive changes of a normal pregnancy begin early in the first trimester and may unmask previously compensated structural heart disease11,12 (Figure 5-1):
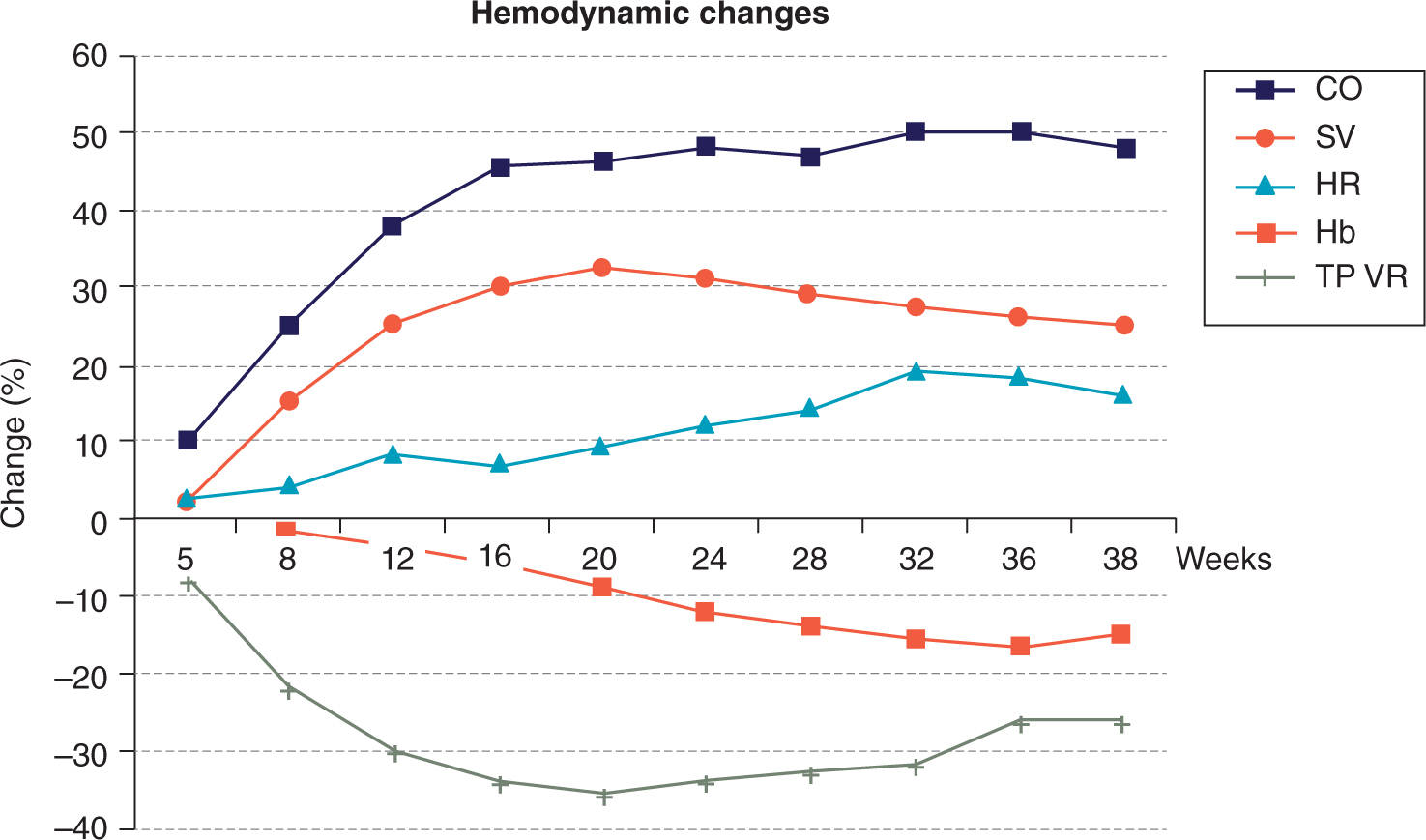
FIGURE 5-1. Hemodynamic changes in pregnancy. CO, cardiac output; SV, stroke volume; HR, heart rate; Hb, hemoglobin; TPVR, total peripheral vascular resistance. Reproduced with permission from Ruys TP, Cornette J, Roos-Hesselink JW: Pregnancy and delivery in cardiac disease, J Cardiol. 2013 Feb;61(2):107-112.
• Increase in plasma volume > red cell mass: physiologic anemia of pregnancy
• Decrease in systemic vascular resistance (SVR) with associated decrease in blood pressure and widened pulse pressure
• Increase in cardiac output (CO) because of an increase in stroke volume and heart rate
• Aortocaval compression after week 20 in the supine position may lead to decrease in CO.
It should be noted that systolic murmurs associated with stenotic valves might be more prominent given the high-flow state of pregnancy. Regurgitant valve murmurs may be lessened with the lower SVR associated with pregnancy. Coagulation is affected in pregnancy with an increase in most of the coagulation factors but also a decrease in fibrinolytic activity. The result is a somewhat hypercoagulable state that develops to reduce the amount of blood loss with delivery. Thromboembolic disease is a very common condition and is one of the most important causes of maternal mortality in developed countries. In addition, obstruction to venous return by the uterus causes stasis and a further rise in risk of thromboembolism.
During labor, an increase in sympathetic tone because of pain and anxiety will cause tachycardia and an increase in SVR. CO may increase by 15% in the first stage and 50% in the second stage of labor.13 Uterine contractions augment both systolic and diastolic blood pressures. Autotransfusion from the uterine circulation occurs immediately after birth leading to an abrupt increase in preload and further increase in CO. Blood loss during delivery may be poorly tolerated in women who are unable to compensate with an increase in heart rate and stroke volume.
MANAGEMENT AND MODE OF DELIVERY
For higher risk patients, invasive hemodynamic monitoring and aggressive medical management may be needed during labor and the immediate postpartum period as these circulatory changes can place significant strain on an abnormal heart. With pain control, cardiac monitoring, and an assisted second stage (to avoid prolonged Valsalva maneuvers), vaginal deliveries can occur safely in most patients with SHD. In general, cesarean delivery is reserved for obstetric conditions, as the preferred mode of delivery for most patients is vaginal. Cesarean delivery may be safer and should be considered for specific patients with structural heart disease4:
• Oral anticoagulation in preterm labor
• Marfan syndrome and aortic diameter >45 mm
• Acute or chronic aortic dissection
• Intractable heart failure.
In addition, cesarean delivery may be advised for those with Marfan syndrome and an aortic diameter 40 to 45 millimeters, severe aortic stenosis (AS), severe forms of pulmonary hypertension (seen in some structural heart disease patients), or acute heart failure. In most of these cases, strict hemodynamic goals should be maintained. No specific structural heart anomaly is an absolute indication for a cesarean section. As stated earlier, most patients may safely deliver vaginally in centers familiar with the care of these high-risk patients. Arterial blood pressure monitoring and central venous access may be needed (for monitoring and possible infusion of vasoactive medications). Because of the associated risks, PA (Swan-Ganz) catheterization is rarely used; however, in patients with heart failure or tenuous hemodynamics, a PA catheter may be indicated and should be placed before active labor or cesarean section.12 A viable alternative is the use of noninvasive CO monitors using either bioreactance or pulse contour analysis. In the postpartum period, cardiac monitoring should continue in higher risk patients. Careful fluid balance is essential to avoid postpartum pulmonary edema in those with poor cardiac reserve because of acute changes in cardiac preload. After delivery, anticoagulation should be re-established, if necessary, and good analgesia maintained to prevent catecholamine surges and tachycardia.
With organized peripartum management, pregnant women with heart disease can have safe pregnancies, undergo labor and delivery, and have acceptable rates of complications. The rates of cesarean section, use of epidural anesthesia, and general anesthesia are similar to the noncardiac obstetric population.14
Type of Anesthesia
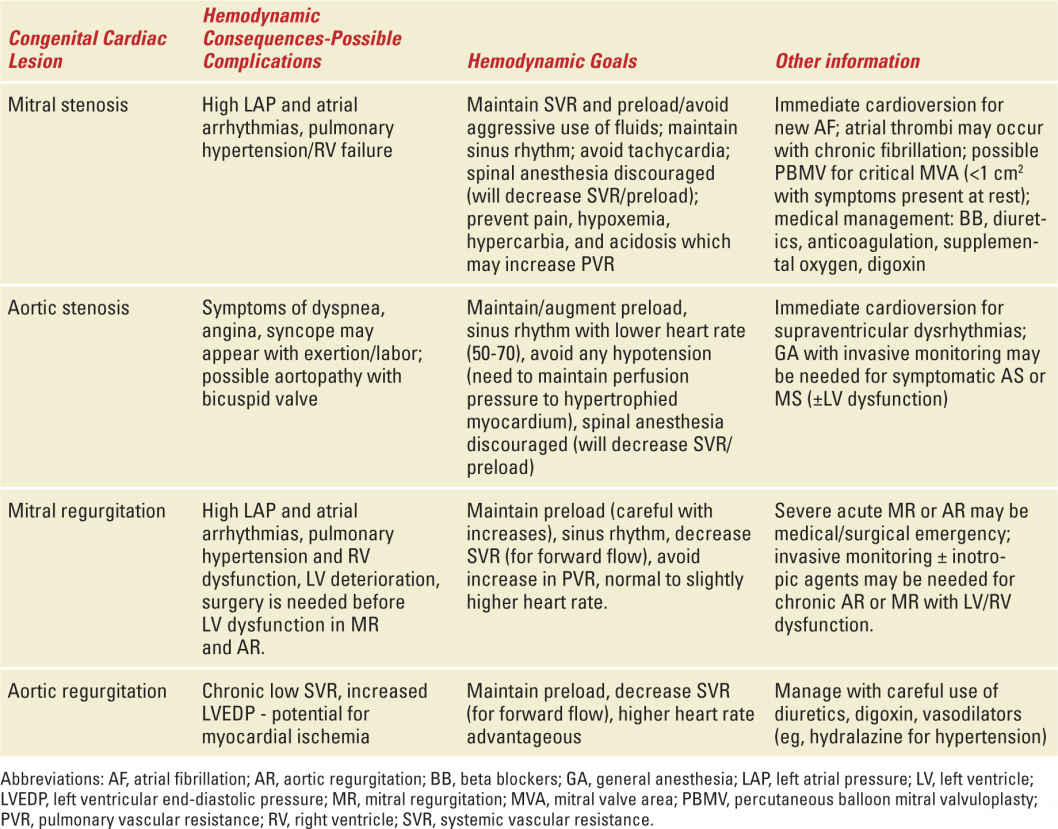
It is important to understand the hemodynamic goals for each specific structural heart lesion before choosing the type of anesthesia for labor and delivery (see Table 5-3). With the exception of emergency cases, general anesthesia should be reserved for maternal cardiac conditions only in specific situations: maternal hemodynamic instability, intractable heart failure with possible respiratory compromise, or inability to tolerate the supine position.14 Most cesarean deliveries can receive regional anesthesia; however, for high-risk NYHA class III or IV patients, slow titration of epidural anesthesia provides more hemodynamic stability than spinal anesthesia. For vaginal or cesarean delivery, continuous lumbar epidural anesthesia is usually optimal; however, preload (volume status) and afterload (blood pressure) conditions must be monitored carefully and maintained. A reduction in SVR seen with regional anesthesia may be poorly tolerated in patients with severe left heart obstruction (such as severe AS and LV outflow obstruction because of hypertrophic cardiomyopathy). Epidural anesthesia is usually well tolerated in those with regurgitant lesions (MR and AR) or left-to-right shunts. The placement of an arterial line with timely use of vasopressors as needed is recommended.
Valvular Heart Disease in Pregnancy |
VALVULAR HEART DISEASE
Valvular heart disease may be diagnosed for the first time during pregnancy when multiple physiologic changes elicit symptoms. Morbidity and mortality for parturients with cardiac valve disease is related to the patient’s functional status before pregnancy.15 Lower risk patients may be managed conservatively with successful outcomes; however, medical measures designed to optimize lesion-specific hemodynamic goals should be used during labor, delivery, and the immediate postpartum periods. Valvular lesions associated with lower maternal and/or fetal risks include the following16:
• Asymptomatic AS with low mean gradient (<25 mmHg and aortic valve area >1.5 cm2) and normal LV systolic function
• NYHA class I or II aortic or MR with normal LV systolic function
• Mitral valve prolapse with no MR or with mild-to-moderate MR with normal LV systolic function
• Mild mitral stenosis (MS) (mitral valve area >1.5 cm2, gradient <5 mmHg) without severe pulmonary hypertension
• Mild-to-moderate pulmonary valve stenosis.
High-risk patients are more likely to have life-threatening maternal or fetal complications. These patients need close surveillance in a specialized tertiary care centers. Clinical deterioration may occur during pregnancy if these valvular heart disease patients also have LV dilation and depressed ejection fraction.17 High-risk patients include16:
• Severe AS with or without symptoms (aortic valve area ≤1 cm2)
• Aortic or MR with NYHA class III and IV symptoms
• MS with NYHA class II to IV symptoms
• Aortic and/or mitral valve disease resulting in severe pulmonary hypertension (pulmonary pressure greater than 75% of systemic pressures)
• Aortic and/or mitral valve disease with severe LV dysfunction (EF less than 40%)
• Mechanical prosthetic valve requiring anticoagulation
• Marfan syndrome with or without AR.
Asymptomatic parturients with regurgitant lesions and normal ventricular function usually tolerate pregnancy well with few complications. In contrast, those with LV stenotic lesions may be unable to adapt to the hemodynamic changes associated with pregnancy.18 Patients with left heart valvular lesions tend to have more complications than those with right-sided lesions. Careful planning and medical therapy in parturients with valvular disease can decrease the prevalence of arrhythmias, ventricular failure with pulmonary edema, infective endocarditis, and thromboembolic complications.
Mitral Stenosis
MS, usually because of rheumatic disease, is the most common clinically significant valvulopathy found in pregnant women.19 Pregnancy may be tolerated in those with mild MS; however, valvular areas <1.5 cm2 may result in significant complications after 30 weeks of gestation and during labor and delivery.20 Symptoms of heart failure may develop immediately postpartum when autotransfusion results in increased preload.15 Several physiologic changes of pregnancy (lower SVR, higher HR, increased preload) may unmask the symptoms of MS by causing an increase in the pressure gradient from the left atrium to the left ventricle. Acutely, this will lead to an elevated left atrial pressure (LAP) and pulmonary venous congestion; also, since forward flow is limited by the stenotic valve, the compensatory increase in CO associated with pregnancy is difficult to achieve and results in an increase in LAP. Tachycardia shortens the LV diastolic filling time and can significantly increase the pressure gradient. Chronic MS causes further increases in LAP, left atrial dilation, possible atrial arrhythmias, and pulmonary congestion; eventually, pulmonary arterial hypertension and right ventricular (RV) dysfunction will develop. Left atrial thrombus development is a concern in those with atrial arrhythmias.
Although the gradient and PA pressures do not correlate with the severity of MS in pregnant patients, they do have some prognostic value.21 Exercise testing before conception can be useful in delineating exercise tolerance and the occurrence of symptoms with an increased CO.4 Medical treatment during pregnancy should focus on decreasing (or avoiding an increase in) the pressure gradient created by the valvular obstruction to LV filling. Initial therapy includes judicious use of diuretics, limitation of salt intake, and avoidance of any strenuous physical activity. Diuretics should be used carefully to avoid decreased uteroplacental perfusion, especially in patients with severe pulmonary hypertension and low CO where excessive diuresis could lead to hypotension.22 Activity restriction and beta blockade can help reduce tachycardia and increase diastolic filling time. A cardioselective beta blocker, such as metoprolol, is a better choice than the nonselective beta blockers.23 Atenolol, in comparison to other cardioselective beta blockers, may be associated with a higher incidence of fetal growth retardation and neonatal bradycardia.12 Given the hypercoagulable state during pregnancy and enlarged left atrium associated with MS, patients with severe MS and left atrial enlargement should be placed on anticoagulation therapy even if MS is not associated with atrial fibrillation.19,24 Pulmonary edema can occur if the patient develops atrial fibrillation with a rapid ventricular rate; prompt treatment with beta-blockers and digoxin is indicated. Refractory atrial fibrillation can be treated with electrical cardioversion.23 Procainamide and quinidine have also been extensively used for suppressive antiarrhythmic therapy during pregnancy.25
Minimally symptomatic patients have less than 1% mortality risk. Morbidity risk can approach 50% in those with NYHA class III or IV symptoms. A 30% fetal mortality may occur if NYHA class IV symptoms coexist with MS.25 The mortality risk increases to 14% to 17% if the patient already has complications of MS such as atrial arrhythmias, thromboembolism, and infective endocarditis.20 If the parturient has moderate or severe MS before pregnancy, she should be counseled against pregnancy and should undergo an intervention, preferably percutaneously, before conception.26
Although ideally performed before conception, percutaneous balloon mitral valvuloplasty (PBMV) is indicated for pregnant women with symptomatic severe MS unresponsive to medical therapy.22,25 Contraindications to PBMV are left atrial thrombi, moderate MR, and subvalvular stenosis.25 PBMV can be performed in the second or early third trimester under echocardiographic guidance or limited fluoroscopy with pelvic and abdominal shielding to minimize radiation exposure.4,22 Although some studies have reported improved fetal outcomes after PBMV during pregnancy, others have shown a high incidence of prematurity, low birth weight, and fetal loss.19 Open mitral valve surgery with cardiopulmonary bypass has been associated with 16% to 33% rate of fetal loss.24,27 Most fetal mortality seems to be associated with high-risk, urgent cardiac surgery and maternal comorbidities at an early gestational age.27
A lumbar epidural combined with vaginal delivery is the usual approach in patients with mild MS or with moderate-to-severe MS with NYHA class I or II heart failure and with PA pressures <50 mmHg.21 Labor can increase LAP and pulmonary wedge pressures by 8 to 10 millimeters mercury. Epidural anesthesia benefits the patient with adequate pain relief and can minimize the intrapartum fluctuations in CO, PA, and LAPs.19 Hypotension associated with epidural anesthesia should be treated with careful titration of crystalloid infusion and vasopressors that are not associated with a chronotropic response (eg, phenylephrine). An assisted delivery device can be used during the second stage of labor to eliminate or decrease the need for pushing. Delivery should be performed in the left lateral upright position.18 The use of tocolytics with beta-mimetic effects should be avoided as the tachycardia associated with these drugs will be poorly tolerated as it will compromise LV filling.19 Monitoring of pulse oximetry, heart rate, and invasive arterial blood pressure is indicated. The use of a Swan-Ganz catheter is controversial secondary to risks of thromboembolic events, arrhythmias, and bleeding complications. However, PA catheters can be helpful in managing hemodynamic changes before delivery and during labor in patients with advanced disease.24 Cesarean section should be performed for obstetric indications or in a patient with severe MS, class III or IV heart failure, and pulmonary hypertension (those in whom PBMV could not be performed).26 A cesarean section can be performed while carefully dosing the epidural, avoiding any decrease in SVR, and immediately treating tachycardia and hypotension with vasoactive medications.
Mitral Regurgitation
Most causes of regurgitant lesions in pregnant patients are rheumatic, congenital, or degenerative. Other causes can include postvalvotomy, endocarditis, or rarely antiphospholipid syndrome. Because pregnancy is associated with a decrease in SVR and LV afterload, MR is usually well tolerated19; especially in asymptomatic patients with preserved LV function.25 Surgery should be reserved for severe worsening MR despite optimal medical therapy.28 If surgery is required, mitral valve repair is preferred to replacement to avoid the need for anticoagulation in the future.16 Patients with moderate-to-severe MR should have exercise testing before conception and are at higher risk of developing heart failure during pregnancy.21 In patients with pulmonary congestion secondary to MR, judicious use of diuretics should be implemented. Digoxin can be used to medically treat LV dysfunction associated with MR.
If the patient has systemic hypertension and valvular regurgitation, vasodilator therapy should be started. Decreasing the patient’s afterload (blood pressure) can reduce the regurgitant volume and promote forward flow of blood. ACE inhibitors are effective afterload reducers, but they are contraindicated during pregnancy because of the teratogenic effects.25 Vasodilators, however, are not recommended in patients with low or normal blood pressure because of the concomitant decrease in preload. Hydralazine and nitrates have been considered a safe drug to use during pregnancy.25 Vaginal delivery is preferable in stable patients, and lumbar epidural anesthesia with shortened second stage of labor is advised.21 Acute decreases in LV function with increased LAP may require inotropic therapy (dobutamine or milrinone) and vasodilator therapy if the blood pressure is elevated.
Aortic Stenosis
Congenital aortic valve disease, such as bicuspid aortic valve, is the most common cause of AS during pregnancy. Because 50% of patients with bicuspid aortic disease and AS also have ascending aorta dilation, aortic measurements should also be taken during imaging studies. Cardiac morbidity is related to the degree of AS, the transvalvular gradient, and symptoms before conception. Most pregnant women with an aortic valve area of >1.0 cm2 who are provided with an early diagnosis and close monitoring can tolerate pregnancy well.19 For patients with AS, it is imperative to maintain normal sinus rhythm, to provide adequate blood pressure and normal heart rate, and to maintain adequate preload. These hemodynamic goals are important for those with mild to moderate AS and normal LV function, as these patients can usually be managed medically during pregnancy. It is important to note that both severe AS and hypertrophic cardiomyopathy with LV outflow tract obstruction poorly tolerate arrhythmias, hypotension, tachycardia, and hypovolemia.
Exercise or stress testing should be considered in asymptomatic patients with moderate to severe AS.16; any elicited symptoms or blood pressure abnormalities may correlate with the ability to tolerate labor and delivery. Symptomatic AS patients should not have exercise testing, as this may worsen or cause dyspnea, syncope, or chest pain. With severe AS in pregnancy (valve area <1 cm2), there is a 10% risk of maternal cardiac complications, including heart failure.28 Fetal risks with moderate to severe AS include preterm labor, intrauterine growth retardation, and low birth weight in up to 25% of parturients.26 An asymptomatic parturient with severe AS or a patient who becomes symptomatic during pregnancy should be optimized with medical therapy: activity restriction, diuretics, oxygen, and beta blockers or nondihydropyridine calcium channel blockers for rate control, especially in the presence of atrial fibrillation.16,29 When symptoms are not alleviated by medical therapy, termination of pregnancy, percutaneous aortic balloon valvuloplasty, or surgery should be considered before labor and delivery.16,25 The rate of fetal loss is less with balloon valvuloplasty than with cardiac surgery, but significant risks include stroke, myocardial infarction, severe AR, and tamponade.29 Patients with severe AS should be followed at least once a month to assess the progression of symptoms and for echocardiographic evaluation.
Vaginal delivery with assisted second stage of labor is preferred in patients with nonsevere AS, and invasive hemodynamic monitoring (central venous catheter and arterial line) should be used during labor and delivery in those with moderate to severe AS.19 Precautions should be taken to avoid any decreases in peripheral vascular resistance associated with neuroaxial anesthesia.21
Aortic Regurgitation
AR in pregnant women is usually because of a bicuspid aortic valve, previous endocarditis, or annulus dilation associated with Marfan syndrome.26 Pre-pregnancy echocardiographic examination should be obtained to evaluate for severity of regurgitation. The decrease in SVR and increased heart rate associated with pregnancy both help decrease the degree of regurgitation. LV failure in conjunction with AR increases the maternal risk, can pose challenges with fluid and blood pressure management during labor and delivery, and may lead to worsening of heart failure during pregnancy.
Parturients with AR can usually be managed conservatively with salt restriction, diuretics, digoxin, and vasodilators.16,26,28 Appropriate vasodilators include nifedipine and hydralazine.25 Surgery may be unavoidable in patients who develop severe, acute regurgitant lesions and severe heart failure refractory to medical management.21 However, asymptomatic patients with severe AR should not undergo prophylactic surgery; they should be optimized medically and will likely tolerate pregnancy well. Vaginal delivery with assisted second stage of labor is preferred in conjunction with epidural anesthesia.18,21 Cesarean section may be considered in those with NYHA class III or IV heart failure, especially if complicated by ventricular dysfunction.29
Pulmonic Valve Disease
Pulmonic valve stenosis usually occurs in conjunction with other congenital heart lesions but can occur by itself. Isolated pulmonic stenosis rarely poses a problem during pregnancy.16 Severe pulmonic stenosis can cause RV failure and arrhythmias. If symptoms develop during pregnancy, balloon valvuloplasty can be considered. This procedure is usually performed under echocardiographic guidance when the peak Doppler gradient across the pulmonic valve is >64 mmHg.21 Pregnant women with severe pulmonic stenosis should have monthly or bimonthly cardiac evaluations, including echocardiographic monitoring for RV dysfunction. Asymptomatic pulmonary regurgitation (PR) may be tolerated well in pregnancy; however, severe PR with RV dysfunction is a high-risk lesion and pre-pregnancy surgical repair is indicated. Vaginal delivery is indicated for mild to moderate pulmonic lesions and NYHA class I or II symptoms. Cesarean section may be considered for parturients with class III or IV heart failure symptoms refractory to medical therapy.
Tricuspid Valve Disease
Congenital tricuspid valve diseases commonly include Ebstein anomaly and tricuspid atresia (see Congenital Heart Disease section). Acquired diseases of the tricuspid valve include endocarditis, myxomatous disease, and carcinoid disease. Most tricuspid regurgitation is functional and secondary to annular dilation because of pressure or volume overload of the RV4 (eg, pulmonary hypertension). Because some of these cardiac conditions involve multiple valve lesions, one must proceed with treatment considering all lesions involved. Pre-pregnancy work-up should include clinical and echocardiographic assessment. Isolated tricuspid regurgitation does not pose significant risk during pregnancy; however, if it is associated with cyanosis or heart failure, the patient should be advised against pregnancy or acquire treatment before conception. Complications with pregnancy associated with tricuspid regurgitation are related to the severity of TR and RV function.21 The preferred mode of delivery is vaginal in almost all cases.
Prosthetic Valves and Anticoagulation
In parturients with prosthetic valves, the successful course of pregnancy and labor depends on preserved LV function, properly functioning valves, and effective anticoagulation.17
The evaluation of pregnant patients with prosthetic heart valves should include a thorough history and physical, focusing on signs of heart failure and functional capacity. An echocardiographic examination of cardiac and valvular function should be performed as well. The history should note the type of prosthetic valve (bioprosthetic/tissue or mechanical), the valvular position, and function of the prosthetic valve. Patients in NYHA class III or IV heart failure before conception should be advised against pregnancy.30 Bioprosthetic (tissue) valves eliminate the need for anticoagulation, but use of these valves will likely lead to reoperation in the future. Half of all porcine tissue valves placed in women of childbearing age will likely fail within 10 years of surgery, especially in the mitral position. Valves placed in the mitral position have a higher risk for thromboembolic events because of the low pressure flow state of the left atrium.31 If the bioprosthetic and LV function is normal, then the maternal cardiovascular risk is low. Women with mechanical heart valves, in contrast, require lifelong anticoagulation to prevent thrombosis. Because of the hypercoagulable state of pregnancy, these parturients are at higher risk for thromboembolic events, with a morality risk of 1 to 4%.30
Counseling women of childbearing age with mechanical heart valves of the risks and benefits of potential therapeutic options to mother and fetus should ensue before conception. Because of the risk of mortality associated with thromboembolic events and prosthetic valves in pregnancy, effective anticoagulation is important. However, there are risks to the mother and fetus with most anticoagulation therapy. Recommendations for anticoagulation on pregnant patients are based on case reports, case series, and extrapolations from nonpregnant patients, as there is a lack of properly designed studies performed in this area. If a pregnant patient with a mechanical heart valve presents with valve thrombosis, the first-line treatment is thrombolysis, if there are no contraindications. As it is associated with a high risk of fetal loss, surgery should only be considered if there is a contraindication to thrombolysis or if thrombolysis is ineffective.30
Vitamin K Antagonists
Vitamin K antagonists (VKA) are the preferred long-term anticoagulation agents in nonpregnant patients; however, during pregnancy, warfarin has been associated with adverse fetal outcomes, such as stillbirth, fetal intracranial hemorrhage, spontaneous abortion, prematurity, and adverse neurological outcomes.32,33 A 6% rate of fetal embryopathy is associated with warfarin usage between 6 and 12 weeks of gestation (usually >5 mg/day dosing).34 Multivariable analysis shows that a daily warfarin dose of greater than 5 mg is associated with poor fetal outcomes, particularly stillbirth.33,35 Warfarin usage during labor has been associated with increased rates of maternal hemorrhage.33 Despite possible complications, these oral anticoagulants are recommended by some experts during the second and third trimesters until the 36th week of pregnancy.4 If women need >5 mg/d warfarin to achieve therapeutic anticoagulation before pregnancy, then it has been recommended to continue the medication throughout the entire pregnancy until the 36th week.36 Women should be counseled about the increased risk of both maternal and fetal complications. For emergency cases in patients receiving warfarin, care should be used when considering reversal warfarin agents, as these may increase thrombosis risk in mechanical valves. Coordination with hematology and cardiology is warranted in emergency cases (such as preterm labor or urgent cesarean section).
Unfractionated Heparin
Heparin is safe for the fetus, as it does not cross the placenta. There have been case reports, however, showing a higher risk of thromboembolic events in pregnant patients with mechanical heart valves managed with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH). These studies included patients with older, more thrombosis-prone valves, inadequate heparin dosing, and poor monitoring of coagulation status.16 Because pregnancy is associated with increased levels of factor VIII and fibrinogen, activated partial thromboplastin time (aPTT) is not as responsive as in nonpregnant individuals.16 Therefore, frequent monitoring of aPTT is necessary. Heparin is associated with increased risk of maternal osteopenia and heparin-induced thrombocytopenia.32
The efficacy of subcutaneous heparin has not been decided and could lead to unpredictable anticoagulation status before labor. In high-risk women with older mechanical valves, the aPTT should be kept at 2.5 times the control value with continuous intravenous infusion of UFH.
Low-Molecular-Weight Heparin
LMWH has been used successfully to treat venous thrombosis in parturients, but there is a lack of data regarding anticoagulation regimens for prosthetic valves. Some advantages of LMWH over UFH include a more predictable dose response, less incidence of heparin-induced thrombocytopenia, and greater ease of administration.16 Because the dose responsiveness is more predictable, the bleeding complications are less with LMWH. Most cases with thromboembolic complications were associated with inadequate dosing, lack of monitoring, or subtherapeutic levels of anti-Xa.32 Because the pharmacokinetics of LMWH are affected during pregnancy, administration of LMWH by weight alone is insufficient. The American College of Chest physicians recommend a peak anti-Xa level measured 4 hours after administration to be about 1 U/mL. Even with peak anti-Xa levels of 0.75 U/mL to 1 U/mL, the associated trough levels of anti-Xa were subtherapeutic at a level less than 0.5 U/mL in most cases. Although vigilance is necessary, good outcomes occur in women are compliant with twice-daily dosing regimens and dose adjustments using anti-Xa levels. Treatment failures (thrombosis of valve) can be minimized by using trough as well as peak anti-Xa levels to adjust the LMWH dose.37 After therapeutic levels are achieved, the postdose (peak) anti-Xa level should be assessed weekly.4
Recommendations for Pregnant Patients with Mechanical Prosthetic Valves
The recommendations for pregnant patients with mechanical prosthetic valves are shown in Table 5-4. For the chosen anticoagulation regimen, close surveillance is important to ensure good maternal and fetal outcomes. Any change in medication regimen should be implemented in the hospital. In addition, echocardiography is immediately indicated in women with prosthetic valves and new onset dyspnea and/or an embolic event. High-risk patients who may require the addition of aspirin include those with LV dysfunction, older prosthetic valves, prosthesis in the mitral position, prior thromboembolic episodes, or atrial fibrillation. Recent recommendations from the American College of Chest Physicians suggest adding low-dose aspirin to the anticoagulation regimen in all patients with mechanical heart valves if they are considered at low risk for bleeding complications. VKA should be discontinued in the 36th week of gestation and dose-adjusted UFH (a PTT ≥2 × control) or adjusted-dose LMWH (target anti-Xa level 4-6 hours postdose 0.8-1.2 U/mL) should be used instead until delivery. LMWH should be replaced by intravenous UFH at least 36 hours before a planned delivery, and UFH should be continued until 4- to 6 hours before planned delivery and restarted 4- to 6 hours after delivery if there are no bleeding complications.4 In the postpartum period, both heparin and warfarin are safe for mothers who choose to breastfeed.
Recommended Anticoagulation Regimens in Pregnant Women With Mechanical Heart Valves (Grade IA) |



