(Creative common license tag by bing) clomiphene citrate
Clomiphene citrate is one of the selective estrogen receptor modulators (SERMs) with both agonistic and antagonistic activities for estrogen receptor. It is a racemic mixture of 62% enclomiphene (Cis-) and 38% zuclomiphene (trans-), the former considered to be the more potent isomere with much shorter half-life (about 24 hours) than zuclomiphene which is less potent, but its elimination from the body may take weeks after a single dose [19], although no clinical or ill effects from zuclomiphene accumulation over repetitive treatment cycles are anticipated [20–22].
Clomiphene use alone for ovulation induction was associated with delayed ovulation or luteinized unruptured follicle development. These issues have been associated with continued estrogen receptor antagonistic effects of enclomiphene at the level of the hypothalamus, which can be mitigated by using human chorionic gonadotropin (HCG) or GnRH agonist to trigger ovulation or final oocyte maturation. If CC is used throughout the stimulation daily rather than limiting the duration solely to 5 days as the norm to induce ovulation in women with chronic anovulation due to polycystic ovary syndrome, then decreased estrogen sensitivity of the hypothalamus may lead to delay of LH surge while allowing for continued multiple follicle growths with continued endogenous FSH action on the ovary.
In one study, the prolonged use of CC at 100 mg daily dose for 15 days resulted in prolonged and continued increase in LH without any LH surge. With continued high levels of LH, some follicles may undergo luteinization without ovulation, which may become an issue. On the other hand, CC use at 100 mg daily dose for 5 days resulted in normal LH levels followed by LH surge [23]. This means that some washout period is required for enclomiphene for proper LH surge to occur. Actually, this effect of CC on LH can be exploited in minimal stimulation protocols for IVF in women with DOR and/or ARA [24].
The use of CC requires intact hypothalamic-pituitary function on the contrary to many conventional stimulation protocols for IVF, which largely rely on hypothalamic suppression with GnRH agonists or GnRH antagonists. Therefore, more physiological contributions of endogenous FSH and LH are ignored in conventional stimulation while relying on recombinant FSH, highly purified human menopausal gonadotropins (HMG) with more reliance on low-dose human chorionic gonadotropin (HCG) for LH activity support, especially in the United States where recombinant LH is not marketed currently. Therefore, conventional stimulation requires higher doses of gonadotropins. In that respect, the prolonged use of CC during a minimal stimulation protocol may result in more physiological stimulation of follicles with less reliance to commercial gonadotropin products. In terms of using gonadotropins, we also prefer alternate day dosing of highly purified HMG rather than the recombinant products.
The CC is started at 100 mg daily dose in minimal stimulation IVF for DOR and/or ARA, unlike using a lower-dose regimen in women with normal to high ovarian reserve who prefer minimal stimulation IVF. In such cases, typical daily dose of CC is 50 mg daily. Our reasoning of using higher doses in DOR and/or ARA patients is differential impact of LH between normal/high ovarian reserve cases and DOR and/or ARA cases. In general, higher LH levels during stimulation are not desirable in women with normal or high ovarian reserve. However, reverse is the case in women with DOR and/or ARA who are believed to be negatively affected from profound LH suppression. Hence, for normal responders, there is an impression that lower LH levels may be well tolerated or even can lead to better pregnancy outcomes [25, 26]. In women with expected POR, however, many conventional IVF stimulation protocols are based on the prevention of LH suppression with protocols like short agonist or micro-agonist dose flare protocols or focusing on appropriate LH activity support which seems to be beneficial in women with DOR and/or ARA [27–29].
12.4 Gonadotropin Co-treatment
Our gonadotropin of choice for minimal stimulation with CC is highly purified human menopausal gonadotropin (hp-HMG). Each vial of hp-HMG contains 75 IU of FSH and 75 IU of LH with some added HCG for additional LH activity support. Each 75 IU vial of hp-HMG contains about 10 IU of HCG [30]. After 4 days of daily CC, hp-HMG is added to CC on day 5 of stimulation and continued on every other day basis until the day of trigger for final oocyte maturation. This choice is based on some data showing beneficial effects of HCG addition to stimulation protocols to assure adequate LH activity support during the stimulation days that GnRH antagonist administration may be needed to prevent premature LH surge. Even with half dose of GnRH antagonist administration, profound LH suppression may be observed in some set of patients, which may result in plateauing E2 levels and slowing down of follicle growth. Theoretically, these unwanted effects may at least be partially mitigated by hp-HMG rather than recombinant FSH use. In conventional IVF stimulation protocols, the addition of HCG to recombinant FSH was found to be beneficial to achieve top-quality embryos [31].
12.5 Preventing Premature Luteinization
In DOR and/or ARA group, keeping the endogenous LH levels at an optimum level is of great importance. The upcoming section will review the control of LH in more detail. GnRH antagonists are mainly used to prevent LH surge during the minimal stimulation IVF cycles. GnRH antagonists promptly and reversibly suppress LH levels as can easily be detected shortly after GnRH antagonist administration due to the known short half-life of LH in the order of minutes [32]. Therefore, GnRH antagonist can be administered whenever it is needed if the serum LH levels are followed closely. The initial dose finding trials of one of the antagonists (ganirelix acetate) used in IVF stimulation protocols revealed that as the dose of daily antagonist increased, more profound suppression of LH was attained, while lower embryo implantation rates were observed. The optimal IVF outcomes were achieved by the 0.25 mg subcutaneous daily dose when it is used at a fixed dose starting on day 7 of stimulation as compared to the other higher dose regimens of 0.5 mg, 1 mg, and 2 mg [33]. As some studies have suggested, more intense dose regimens of GnRH antagonist may affect IVF outcomes at an adverse fashion, and GnRH antagonist may have some direct effects on the ovaries at granulosa cell level [34–36].
Considering the information as summarized above, we start monitoring E2 and LH levels as early as day 4 of stimulation in order to assess when GnRH antagonist would be required to prevent premature LH surge. Clomiphene citrate usually induces some LH flare in DOR and/or ARA patients, but again unless the level of LH is close to or above 10 IU/L while E2 levels are at or above 200 pg/mL, GnRH antagonist administration is not required. When needed, it is usually preferred at 1/2 of the suggested daily dose. Since the 0.25 mg cetrorelix acetate vial can be used in half doses without wasting the other half dose of the medication, we prefer cetrorelix acetate use. At least in the United States, ganirelix acetate is marketed in individual prefilled fixed needle syringes, and therefore with half dose use, the other half has to be wasted which will increase the cost of the treatment cycle for the patient.
12.6 Trigger for Final Oocyte Maturation
Once the E2 levels reach at or above 250 pg/mL, and the LH levels are maintained at less than 10 IU/L or more than 2 IU/L with appropriate administration of half dose cetrotide as needed, the E2 progression along with follicle growth rate is closely observed. The trigger agents are considered when the follicle sizes are between 16 and 20 mm in average diameters according to the patient factors and information obtained from the recent IVF cycles of some patients with such data available. Especially in profound DOR cases and DOR cases at or above 40 years of age, spontaneous follicle collapse shortly after trigger for final oocyte maturation can be a problem. In such cases if the leading follicle size is >18–19 mm, a nonsteroidal anti-inflammatory agent administration the day after trigger can be considered as we will discuss in another chapter.
Deciding on the leading size of the follicle to administer the trigger agent depends on many factors and highly customized for each patient. In women with ARA, at or above 43 years of age, it was recommended that the lead size of the follicles should be between 16 and 18 mm to minimize premature luteinization of granulosa cells in this population [37]. This was related to aging-induced aberration in proper proliferation of granulosa cells as they respond to high levels of FSH typically found in this patient population. Granulosa cell luteinization may be a reflection of the arrest in granulosa cell proliferation, one step away from apoptosis. This phenomenon may be associated with poor oocyte and embryo quality leading to lower pregnancy rates [38]. Although minimal stimulation is typically performed to avoid high levels of FSH and if the endogenous FSH is found high, resorting to other mild stimulation approaches is considered; with staggering E2 progression while noticing mildly elevated levels of progesterone with normal (<10 IU/L) LH levels, we do consider triggering between 16 and 18 mm size of the leading follicle if there are no other growing follicles below 16 mm. Otherwise, we can still proceed with trigger with a lead follicle size of 19–20 mm. We document the follicle sizes corresponding to oocyte yield in patients to assist with future trigger decisions for their subsequent minimal stimulation cycles.
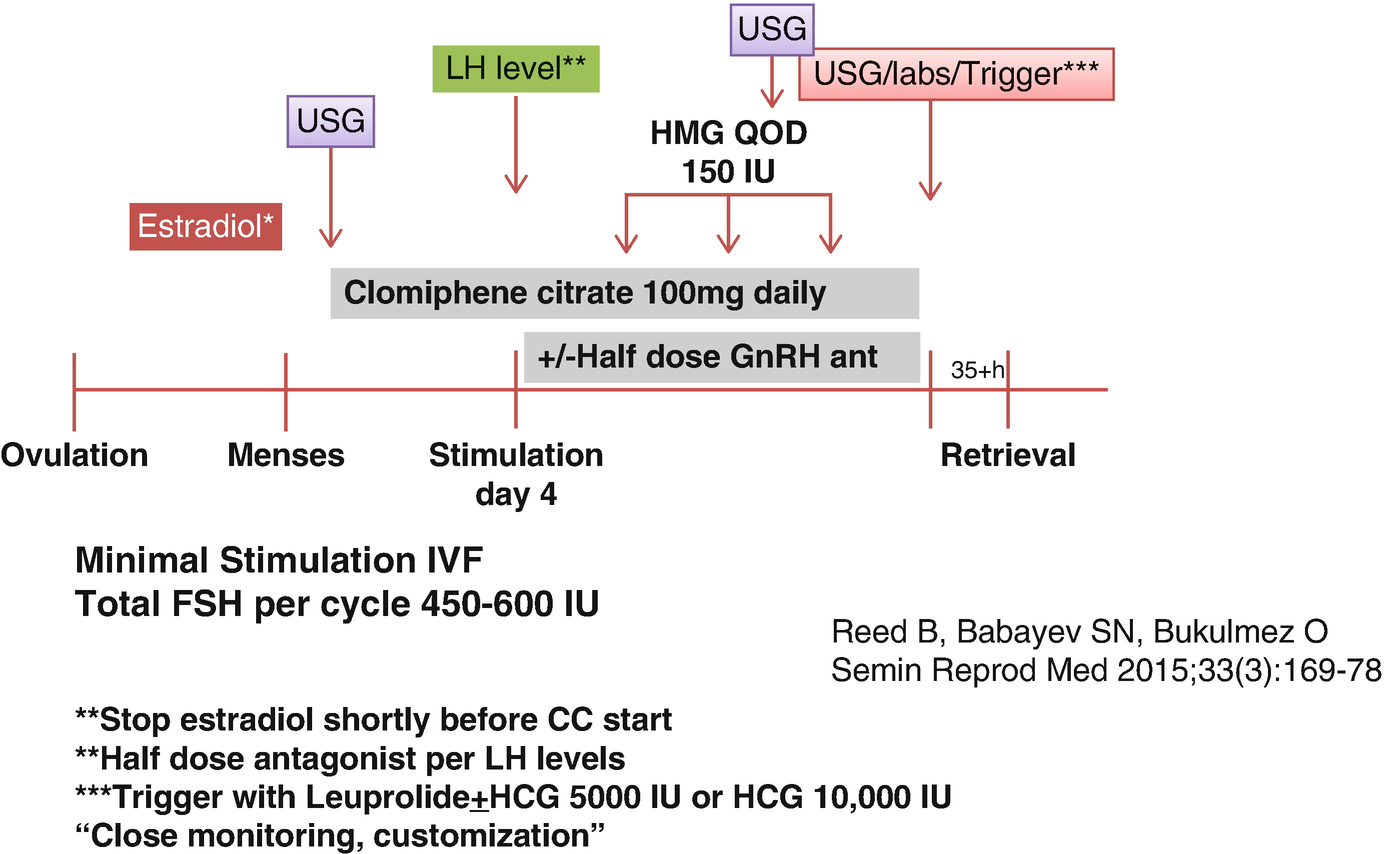
Minimal stimulation protocol with clomiphene citrate
12.7 Considerations for Oocyte Retrieval, Fertilization, and Embryo Freezing
Since still the number of oocytes is an important parameter for the number of embryos developed for future frozen embryo transfers, the method used for oocyte retrieval is important. All retrievals are performed under general anesthesia without intubation. We will have another section on oocyte retrieval in minimal and mild stimulation protocols for DOR and/or ARA patients. Briefly, we use 17G single lumen oocyte retrieval needle with the tubing extending toward the collection or sampling tube (K-OSN-1735-B-90-US, Cook Medical, Brisbane, Australia). We attach the tubing to the Rocket Craft R29655 suction pump (Rocket Medical plc, Washington, Tyne &Wear, NE38 9BZ, England). We keep the pressure at around 120 mm Hg never to exceed 180 mm Hg. The single lumen needle can also be used to flush the follicles by using the opening within the silicone rubber cork of the sampling tube by injecting warmed sterile media flush toward the needle with a 10 cc syringe while minimizing air within the tubing.
Follicle flush has been debated over several decades. The systematic review of five randomized controlled trials comparing follicular flushing to aspiration only in general IVF population suggested comparable number of oocytes and the clinical pregnancy and live birth rates (only one trial reported) between the groups. As expected, the duration of the oocyte retrieval is prolonged with flushing [39]. The quality of evidence was admitted to be moderate, and the results may show some imprecision per authors. Another recent Cochrane review also reported similar results from ten studies in general IVF population [40]. One study involving only minimal stimulation IVF cycles for women with POR demonstrated that, with only follicle aspiration alone, the oocyte recovery rate could be 46.8%, while with follicular flushing this rate may be increased to 84.6%. The same study even suggested that the oocytes retrieved with follicular flushing result in a better morphology and implantation rates than the oocytes readily recovered with first attempt of aspiration without flushing [41]. Consideration for follicular flushing is an essential element of minimal/mild stimulation and natural cycle approaches for DOR and/or ARA patients who typically present with limited number of follicles.
There will be a discussion in the upcoming chapters that the fertilization method for the oocytes, conventional insemination versus intracytoplasmic sperm injection (ICSI), should carefully be thought over. Although there is a tendency to use ICSI for the majority of ART cycles, conventional IVF may actually be considered in many women with DOR and/or ARA due to many physiological differences between these two fertilization methods [42].
Then the stage of the embryo considered for cryopreservation also depends on many factors. The information obtained from multiple minimal stimulation cycles, whether preimplantation testing for aneuploidy is desired or not, are important components of decision-making. Minimal stimulation approach for women with DOR requires accumulation of either day 3 or day 5/6 stage frozen embryos in appropriate grade for their stage to maximize success in their future frozen-thawed embryo transfer (FET) cycles. Frozen embryo transfer preparation should not be taken lightly as well. In patients with reliable menstrual cycles, natural cycle preparation can be considered especially if they show erratic response or compliance issues with oral or transdermal estradiol preparations that we will review in another chapter.
12.8 Aromatase Inhibitor Use for Minimal Stimulation
Letrozole is an oral aromatase inhibitor. Letrozole, by inhibiting conversion of androgens to estrogens by follicular granulosa cells and showing the same effects in the brain, will lead to increased gonadotropin release via a central mechanism somewhat similar to clomiphene citrate. Letrozole has a shorter half-life (about 45 hours) than CC, and it does not deplete estrogen receptors unlike CC. Letrozole may also have some proposed benefits in increasing FSH sensitivity while promoting early follicular growth due to increase in intraovarian androgens [43].
Letrozole use for ovulation induction and controlled ovarian stimulation had been impacted negatively by an abstract reporting increased risk of especially locomotor and cardiac defects in the babies conceived via letrozole or letrozole with gonadotropins [44]. Although various larger and better designed studies did not confirm any such findings, letrozole use in ovulation induction or other ovarian stimulation protocols stayed off-label. Recently, after critical analysis of studies based on letrozole use in ovulation induction for women with polycystic ovary syndrome (PCOS), the American College of Obstetricians and Gynecologists affirmed that letrozole, rather than CC, should be the first-line therapy for ovulation induction due to the increased live birth rate when compared to CC, also considering the recent safety data on letrozole [45].
Aromatase inhibitors like letrozole has been used in some conventional stimulation protocols for IVF to enhance cycle outcomes in women with expected poor ovarian response (POR). These studies involved using 5 days of letrozole in the beginning of stimulation for 5 days along with a high-dose gonadotropin protocols. These studies suggested some partial benefits of letrozole addition to gonadotropins as compared to those not using letrozole or those using GnRH agonist microdose flare protocol [46, 47]. A recent Cochrane review analyzed if letrozole or CC use with or without gonadotropins makes any difference in IVF outcome. Most of the studies included in this review actually included poor responders, and many were about letrozole co-treatment in this patient population. In general IVF patient population, live births or clinical pregnancy rates did not significantly change with letrozole or CC use with or without gonadotropins as compared to gonadotropin use with GnRH analogs. The addition of these oral agents though was associated with decreased risk of ovarian hyperstimulation syndrome. In poor responders, the conclusions on live births and clinical pregnancy rates did not change in terms of CC or letrozole use with or without gonadotropins versus gonadotropins with GnRH analogs. There was a moderate quality of evidence that co-treatment with CC or letrozole might decrease the mean gonadotropin dose. However, their use with gonadotropins may be associated with the increased cycle cancellation rate and decreased number of oocytes retrieved in both general IVF population and in women with POR [48].
The context of letrozole use is different from its use in conventional stimulation protocols where letrozole use is limited to 5 days with the daily doses ranging from 2.5 to 5 mg mostly with the intention of proceeding with fresh embryo transfer. It is known that further increase in daily dose of letrozole higher than 7.5 mg may result in thin endometrial lining as observed in CC [45, 49]. Also within the context of superovulation with intrauterine insemination for unexplained infertility, letrozole showed lower clinical pregnancy and live birth rates than CC and gonadotropins alone, although multiple gestation rates between letrozole and CC were comparable [50]. The authors suggested some potential mechanisms for such differences including the differential effects of letrozole on the endometrium, ovary, and central nervous system different from those with CC in unexplained infertility patients.
Letrozole use in minimal stimulation is similar to CC use. It is prescribed for everyday use at daily doses between 2.5 to 5 mg, while adding hMG 150 IU every other day on day 5 of stimulation. The use of letrozole daily until the day of triggering for final oocyte maturation during controlled ovarian stimulation is not a new concept and has been implemented in patients with estrogen receptor-positive breast cancer undergoing embryo or oocyte freezing cycles [51]. Using daily letrozole at 5 mg daily dose along with daily gonadotropin doses ranging from 150 to 300 IU resulted in much lower peak estradiol levels with decreased gonadotropin dose requirements as compared to historical controls with tubal factor infertility who underwent conventional IVF stimulation without daily letrozole, while keeping the fertilization and embryo development rates comparable if the letrozole arm is triggered with a leading follicle size of ≥20 mm rather than with the follicle size of ≥17–18 mm [52]. The reason for this increased diameter threshold for letrozole-treated cycles is the perception of decreased oocyte maturity rate. Even using this threshold, a compromised oocyte maturation with daily letrozole use was reported, and some authors demonstrated decreased fertilization rates and increased gonadotropin requirements with letrozole co-treatment in breast cancer patients as compared to age-matched infertile controls [53, 54]. One retrospective cohort multicenter study from Italy compared the oocyte cryopreservation outcomes between estrogen receptor-positive and estrogen receptor-negative breast cancer patients. Those with estrogen receptor-positive tumors received letrozole co-treatment with gonadotropins and demonstrated significantly decreased mean number of oocytes than the estrogen receptor-negative group treated only with gonadotropins. Letrozole co-treatment arm showed lower gonadotropin requirements to achieve the same number of developing follicles as the patients only treated with gonadotropins, while ending up with lower peak levels of estradiol. The authors argued whether scientific evidence was there to use letrozole co-treatment in estrogen receptor-positive breast cancer patients since mature oocyte yield may be 40% lower with letrozole co-treatment [55]. Certainly, there may be many biases in each of these retrospective reports, and the stimulation response may be different per estrogen receptor status and in breast cancer patients in general as compared to those with infertility [56].
Within the context of mild stimulation, most studies used letrozole in a classic 5-day course combined with daily gonadotropins at 150 IU daily. A retrospective study compared outcomes of mild stimulation protocol with 5-day courses of either letrozole 5 mg daily or CC 25 mg daily combined with HMG commenced at 75–150 IU daily dose started on day 3 of letrozole or CC treatment [57]. Pretreatment programming was made by using oral contraceptives. Patients with DOR were encouraged to try these protocols, and those with high ovarian reserve were excluded. Ovulation trigger was achieved with hCG 10,000 IU when the lead follicle was ≥17 mm, and the oocyte retrieval was performed 34 hours after. All follicles at or above 12 mm were accessed, and all follicles at or above 14 mm in size were flushed multiple times. Peak mean E2 level was significantly lower in the letrozole-treated group as compared to those treated with CC (516 versus 797 ng/mL). CC group produced significantly more mature oocytes (3.3 versus 2.4). All embryo transfers were performed on the third day after oocyte retrieval. CC co-treated group resulted in more mean number of embryos transferred than the letrozole arm (2.5 vs 1.5). Endometrial thicknesses between the groups were comparable. Live birth rates per embryo transfer were also comparable between letrozole (17.7%) and CC co-treated (21.4%) groups. Therefore, letrozole and CC co-treatment seemed to have comparable live birth outcomes with fresh transfers. Again the immature oocyte yield was higher in the letrozole arm [57].
With the daily use of letrozole, estradiol monitoring is less reliable, and at times due to low E2 levels, LH surge may not be observed, and low-dose GnRH antagonist may be required somewhat less frequently. However, continuous letrozole use may have less LH flare effects than CC, which may not work for some DOR and/or ARA patients. We prefer CC primarily for minimal stimulation for DOR and/or ARA patients. Letrozole is considered in women who cannot tolerate CC, who may have activation risk for an autoimmune or inflammatory condition with CC or those with history for estrogen receptor-positive breast cancer. In patients who did not have satisfactory outcomes with minimal stimulation using CC rather than switching to letrozole, we recommend other mild stimulation alternatives, like luteal phase mild stimulation. We still recommend freezing all the embryos for minimal stimulation with daily letrozole due to the frequent observation of thin and non-trilaminar endometrial stripe pattern with the letrozole protocol.
12.9 Costs of Minimal Stimulation Versus Conventional High-Dose FSH Protocols for Diminished Ovarian Reserve Patients

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


