Luteal phase mild stimulation with a recombinant FSH
Oocyte retrieval is scheduled 35–35.5 hours after the trigger. Frequently, such women may start their menstrual bleeding at around the day of oocyte retrieval. We did not find this detrimental to the cycle outcome, and we do not use prophylactic antibiotics during the oocyte retrievals routinely. Having menstrual bleeding during the oocyte retrieval is not one of the indications for antibiotic prophylaxis at our center.
Similar to minimal stimulation cycles, we freeze embryos to provide the best chances for frozen-thawed embryo transfers in the future. In cases, requiring embryo biopsy for genetic testing, we have to watch them in culture until they reach the blastocyst stage. In other cases, we attempt to freeze stage-specific embryos to later synchronize the endometrium for either day 3 or day 5 stage. Like reported by others, we freeze all top-quality eight-cell stage embryos on day 3. Other embryos are taken toward the blastocyst stage for vitrification [15, 16].
13.4 Prolonged Ovarian Suppression and Estrogen Priming Followed by Mild Stimulation
This option is reserved as a last resort protocol for women with profound DOR within the spectrum of premature ovarian insufficiency (POI). These women show high basal FSH above 40 IU/mL and short cycles with short follicular and luteal phases. It is very frequent to observe multiple cystic follicles with increased E2 levels, which is typical for a pre-menopausal state. In such women, obtaining competent oocytes with minimal stimulation or luteal phase mild stimulation would not be possible.
As we discuss in the chapter on activation of ovarian cortex, prolonged suppression of both FSH and LH in such women still with some oocytes in their ovaries may be beneficial for natural stimulation of early folliculogenesis. The clinicians performing many medicated frozen-thawed embryo transfer cycles with ovarian suppression followed by estradiol use may note that after 2 weeks of GnRH agonist suppression followed by 12–14 days of estradiol treatment to develop the endometrial stripe, emergence of 2–6 mm antral follicles can be observed on the day of assessment of the endometrial lining before starting progesterone. Since minimal and mild stimulation protocols require accumulation of frozen embryos, we perform quite few frozen-thawed embryo transfers. We are inspired by observing increased 2–6 mm antral follicles in the ovaries of many women with DOR while performing their transvaginal ultrasounds primarily to make decisions about their endometrial stripe thickness and pattern. That was the reason that we called this mild stimulation alternative as FET (for frozen embryo transfer) protocol informally to be on the same page with our team members.
It has been shown that prolonged suppression of FSH and LH by using GnRH analogs can be useful in women with hypergonadotropic amenorrhea and also those with POI of genetic origin in terms of achieving spontaneous ovulation or response to gonadotropin treatment, respectively [17, 18]. Suppression may last from 4 to 12 weeks followed by estradiol use for priming of follicles. This approach may decrease the forced early follicle selection by high FSH and may restore responsiveness of follicles to FSH when needed perhaps through mitigation of both FSH and LH receptor desensitization at granulosa cell level. LH suppression may also relieve medullary pressure to the cortex via its ovarian stromal effects, which will be discussed in activation of ovarian cortex chapter.
13.4.1 Treatment Details
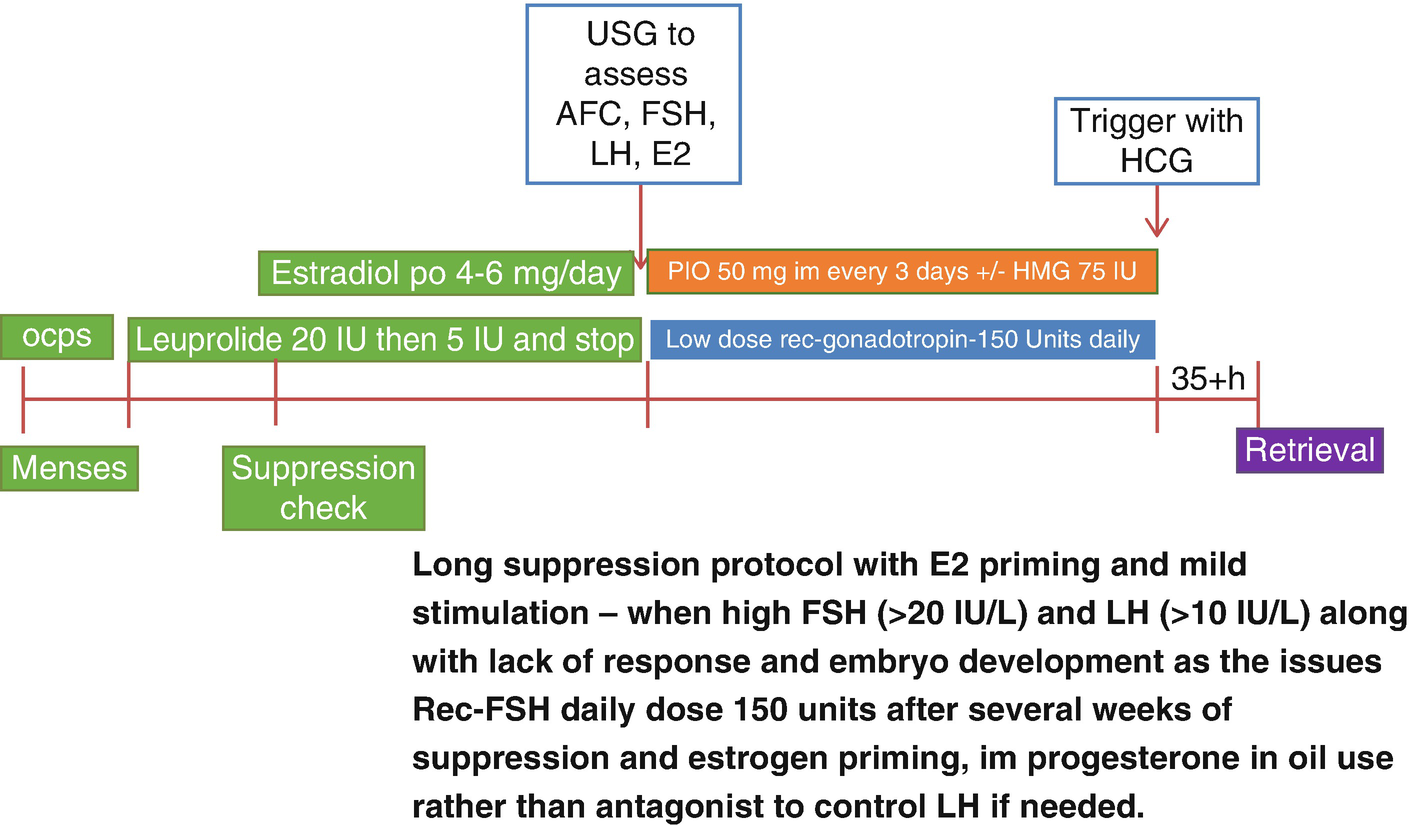
Prolonged ovarian suppression, estrogen priming, and mild stimulation protocol
We recommend this protocol in women younger than 40 years of age within the POI spectrum in terms of FSH levels and mostly undetectable AMH levels but still having some menstrual cycles. Menstrual cycles can either be spaced apart more than every 35 days or may be more frequent than every 24 days.
If there is no contraindication, patients start using combined birth control pills (OCPs) containing at least 30 mcg of ethinyl estradiol with the first day of their menses (cycle day 1 or 2). After about 8–10 days of OCP use, patients need a transvaginal ultrasound to assess for potential follicle cyst presence and laboratory tests to assess E2 and LH levels to start GnRH agonist, leuprolide acetate 20 IU daily. If they have E2 levels at or above 100 pg/mL combined with LH levels at or above 5 IU/L while seeing follicle(s) >8 mm, we delay starting GnRH agonist. If we start GnRH agonist with such findings, due to the initial flare effects of GnRH agonist, follicle cyst formation may happen. At times, these cysts are functional, and they may take up to 3 months for them to resolve. Therefore, it would be best to make sure that OCPs are showing at least some LH suppressing effects before we start GnRH agonist. In women with contraindications to use OCPs, we try oral medroxyprogesterone acetate 10–20 mg daily.
After 7–10 days of daily GnRH agonist use, OCPs are stopped, and patients continue with only leuprolide acetate 20 IU (1 mg) daily with the goal of suppressing LH to less than 1 IU/L. At that point, E2 will be suppressed to below 20 pg/mL unless patients develop functional ovarian cyst or follicle growth. Once achieved, the suppressed state may be maintained for another 2–4 weeks or longer while assessing the ovaries and E2 and LH levels weekly. Then when the emergence of 2–4 mm antral follicles are observed, oral daily micronized estradiol is started to keep E2 levels at 150 pg/mL or above to assure continued FSH suppression when the leuprolide acetate dose is dropped to 5 IU daily. Twice weekly transvaginal ultrasounds and E2 and LH levels are performed to assess further emergence of antral follicles 2–6 mm in size. When these are observed, both micronized estradiol and GnRH agonist are discontinued, and recombinant FSH at 150 IU daily dose is started. Transvaginal ultrasound and E2 and LH monitoring are started after the first 3 days of stimulation. When increasing LH levels >4 IU/L are observed, in order to avoid GnRH antagonist use and just like a frozen-thawed embryo transfer preparation, progesterone in oil injections (PIO) 50 mg intramuscularly every 3–4 days are started titrating to P4 and LH levels. Progesterone levels are kept at or above 3 ng/mL to prevent LH surge. Many patients may not need PIO at all, or all they need may be one to three doses.
Some patients do not show recovery of LH levels during the stimulation phase. If the endogenous LH levels are continuing to be below 1 IU/L, such women may need addition of hp-HMG to the protocol. Either recombinant FSH is decreased to 75 IU, and 75 IU daily HMG is added, or at times especially if FSH levels are also below 8 IU/L, 75 IU of HMG is added to the daily 150 IU dose of recombinant FSH. Although this may seem to be a compromise from the principles of mild stimulation, monitoring of FSH levels may help with this decision-making.
When the leading follicle size of 16–18 mm is reached, final oocyte maturation is triggered with HCG 5000–10,000 IU, and oocyte retrieval is performed at 35–35.5 hours. As in all minimal and mild stimulation cycles, fertilization method of choice (conventional IVF or intracytoplasmic sperm injection) and the embryo stage at vitrification are individualized per patient.
After the oocyte retrieval, since the patients show endogenous P4 increase, we offer an assessment of the ovaries in 2–3 days to start luteal phase mild stimulation if we see more 2–6 mm antral follicles. Therefore, we use the principles of dual or double stimulation in this protocol to take advantage of emergence of antral follicles. Unlike others, we do not start stimulation on the day or day after oocyte retrieval since we use HCG to trigger for final oocyte maturation especially in cases with P4 levels are still >3 ng/mL on the day of trigger or those with suppressed LH levels. We had witnessed that some antral follicles observed during the oocyte retrieval may just disappear 2–3 days after the oocyte retrieval suggesting that some may just be atretic follicles.
As we discuss in the activation of ovarian cortex chapter, using GnRH antagonist for prolonged suppression phase may have some additional merits. Currently the use of daily GnRH antagonist for prolonged suppression may increase the medication costs considerably. However, with the recent marketing of oral antagonist like elagolix, GnRH antagonist can be used for this indication in the near future.
13.5 Conclusion
In women with profound DOR associated with FSH increase above 20 IU/L at basal state or as tested during the minimal stimulation cycle, we recommend additional approaches to obtain competent oocytes for frozen embryo accumulation. These approaches are through luteal phase mild stimulation or prolonged suppression-estrogen priming-mild stimulation protocols as discussed in this chapter. Further refining of such protocols would be possible in the future by the use of medroxyprogesterone acetate to avoid LH surge when the luteal phase is short or by the use of oral antagonist to prevent flare effects of GnRH agonists.

Full access? Get Clinical Tree


