Chapter 174 Staphylococcus
174.1 Staphylococcus aureus
Etiology
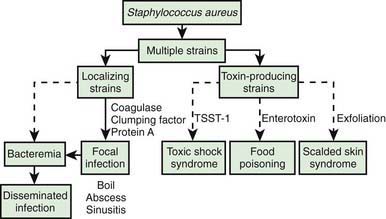
Many strains of S. aureus release 1 or more exotoxins. Exfoliatins A and B are serologically distinct proteins that produce localized (bullous impetigo) or generalized (scalded skin syndrome, staphylococcal scarlet fever) dermatologic complications (Chapter 651). Exfoliatins produce skin separation by splitting the desmosome and altering the intracellular matrix in the stratum granulosum.
Pathogenesis
The development of staphylococcal disease is related to resistance of the host to infection and to virulence of the organism (Fig. 174-1). The intact skin and mucous membranes serve as barriers to invasion by staphylococci. Defects in the mucocutaneous barriers produced by trauma, surgery, foreign surfaces (sutures, shunts, intravascular catheters), and burns increase the risk for infection.
Clinical Manifestations
Respiratory Tract
Infections of the upper respiratory tract due to S. aureus are rare, in particular considering the frequency with which the anterior nares are colonized. In normal hosts, otitis media (Chapter 632) and sinusitis (Chapter 372) are rarely caused by S. aureus. S. aureus sinusitis is relatively common in children with cystic fibrosis or defects in leukocyte function and may be the only focus of infection in some children with toxic shock syndrome. Suppurative parotitis is a rare infection, but S. aureus is a common cause. A membranous tracheitis that complicates viral croup may be due to infection with S. aureus, but other organisms are also possible. Patients typically have high fever, leukocytosis, and evidence of severe upper airway obstruction. Direct laryngoscopy or bronchoscopy shows a normal epiglottis with subglottic narrowing and thick, purulent secretions within the trachea. Treatment requires careful airway management and appropriate antibiotic therapy.
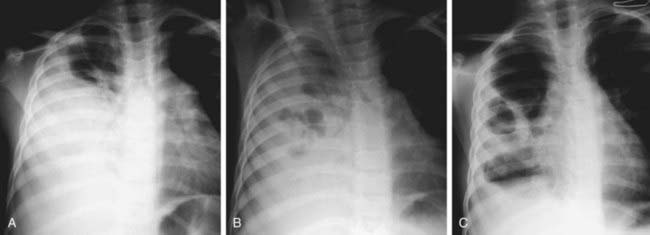
Pneumonia (Chapter 392) due to S. aureus may be primary (hematogenous) or secondary after a viral infection such as influenza. Hematogenous pneumonia may be secondary to septic emboli from right-sided endocarditis or septic thrombophlebitis, with or without the presence of intravascular devices. Inhalation pneumonia is caused by alteration of mucociliary clearance (see cystic fibrosis, Chapter 395), leukocyte dysfunction, or bacterial adherence initiated by a viral infection. Common symptoms and signs include high fever, abdominal pain, tachypnea, dyspnea, and localized or diffuse bronchopneumonia or lobar disease. S. aureus often causes a necrotizing pneumonitis that may be associated with development of empyema, pneumatoceles, pyopneumothorax, and bronchopleural fistulas.
Bones and Joints
S. aureus is the most common cause of osteomyelitis and suppurative arthritis in children (Chapters 676 and 677).
Central Nervous System
Meningitis (Chapter 595.1) caused by S. aureus is not common; it is associated with penetrating cranial trauma and neurosurgical procedures (craniotomy, cerebrospinal fluid [CSF] shunt placement) and less frequently with endocarditis, parameningeal foci (epidural or brain abscess), diabetes mellitus, or malignancy. The CSF profile of S. aureus meningitis is indistinguishable from that in other forms of bacterial meningitis.
Heart
S. aureus is a common cause of acute endocarditis (Chapter 431) on native valves. Perforation of heart valves, myocardial abscesses, heart failure, conduction disturbances, acute hemopericardium, purulent pericarditis, and sudden death may ensue.
Kidney
S. aureus is a common cause of renal and perinephric abscess (Chapter 532), usually of hematogenous origin. Pyelonephritis and cystitis due to S. aureus are unusual.
Toxic Shock Syndrome (TSS)
S. aureus is the principal cause of TSS (Chapter 174.2), which should be suspected in anyone with fever, shock, and/or a scarlet fever-like rash.
Intestinal Tract
Food poisoning (Chapter 332) may be caused by ingestion of preformed enterotoxins produced by staphylococci in contaminated foods. Approximately 2-7 hr after ingestion of the toxin, sudden, severe vomiting begins. Watery diarrhea may develop, but fever is absent or low. Symptoms rarely persist longer than 12-24 hr. Rarely, shock and death may occur.
Diagnosis
Differential Diagnosis
Skin lesions due to S. aureus may be indistinguishable from those due to group A streptococci; the former usually expand slowly, while the latter are more prone to spread rapidly. S. aureus pneumonia can be suspected on the basis of chest roentgenograms that reveal pneumatoceles, pyopneumothorax, or lung abscess (Fig. 174-2). Fluctuant skin and soft tissue lesions also can be caused by other organisms, including Mycobacterium tuberculosis, atypical mycobacteria, Bartonella henselae (cat-scratch disease), Francisella tularensis, and various fungi, among others.