Sleep Disorder Breathing
A Dental Perspective
Introduction
With their 1981 publication Western Diseases: Their Emergence and Prevention,1 authors Hugh Trowell and Denis Burkitt essentially launched a new paradigm in medical education; many modern diseases are now better understood when viewed from an evolutionary perspective. As healthy circadian sleep cycling during childhood would have been absolutely necessary for our ancestors’ survival and reproduction (collectively referred to as evolutionary fitness) over the vast time span of human evolutionary history,2 sleep-related breathing disorders such as obstructive sleep apnea (OSA) were likely not a part of the human experience until fairly recently, and thus can be appropriately categorized as Western diseases (WDs).
Evolutionary medicine (EM), also known as Darwinian medicine,3 is a new approach providing a useful framework for understanding modern diseases from an evolutionary perspective. Evolutionary oral medicine (EOM), or Darwinian dentistry, describes how EM principles can be applied to exploring the evolutionary basis of modern dentofacial maladies such as dental caries, periodontal disease and malocclusion. For example, one proposed explanation by EM/EOM proponents for why humans have only recently begun to become vulnerable to many modern diseases such as type 2 diabetes and dental malocclusion, for example, is the Mismatch hypothesis,4 which postulates that current high prevalences of WDs in industrialized populations are due, at least in part, to exposure to modern feeding regimens and environmental conditions which are vastly dissimilar, or mismatched to, the Paleolithic/pre-agricultural diets and environments to which the human genome has been best adapted.5
Pediatric sleep-disordered breathing (SDB) is a pathological condition associated with a wide range of clinical symptoms, historical evidence, dentofacial physical examination findings, environmental components and genetic and/or epigenetic factors. Recently published controlled studies indicate a close association between pediatric SDB/OSA and neurocognitive impairments such as ADD/ADHD and other behavioral disorders.6,7 Many of the various physical characteristics associated with high prevalences of pediatric SDB/OSA are also strongly associated with a number of pediatric dentofacial abnormalities; the relationship between pediatric SDB/OSA and the developing jaws and facial structures is also well described.8,9
Many craniofacial traits are, for the most part, alterable during the early childhood stages of dentofacial growth and thus likely to play a large role in the presence or absence of clinical symptoms associated with pediatric SDB/OSA and its associated clinical morbidities. Consequently, the role of the orthodontist, pediatric dentist and general dentist as an integral member of every child’s comprehensive healthcare team has never been more important.10,11
Etiology of Malocclusion
Anthropological studies confirm that dentofacial malocclusion (poorly aligned jaws and teeth), a known risk indicator of SDB/OSA,12 was infrequently suffered by our pre-industrial ancestors, and seldom occurs with frequency in extant non-Westernized aboriginal cultures.13 In fact, skeletal malocclusion didn’t appear appreciably in humans until around the time of the Industrial Revolution of the mid-eighteenth century,14 and wherever occasionally observed before that era, it was usually confined to privileged-class individuals.15
In order to most efficiently address the health problems known to be associated with untreated, and/or inappropriately treated malocclusion, it would first be helpful to have some idea about why our fairly recent ancestors seldom suffered from these unpleasant dentofacial, dental and skeletal disharmonies. Anthropologists have understood for decades that human craniofacial volume has been steadily diminishing since around the time of the Agricultural Revolution some 10, 000–12 000 years ago, and most rapidly over the past 350–400 years.14 While there seems to be a definite observable trend towards increased prevalences of malocclusion over the last three to four centuries, to date there is not yet firm consensus amongst dental anthropologists as to precisely what happened, but there does seem to be a growing body of evidence that seems to suggest that feeding behaviors during infancy and early childhood are likely involved.16 Specifically, ancestral-type breastfeeding and weaning are known to be protective against certain forms of malocclusion,17 likely due to the physical challenges posed to the developing palatal–facial suture complex (P–FSC) during infancy and early childhood; furthermore, the highly processed/soft baby foods and artificial infant formulas/commercial nipples that are in so much use today were simply not readily available to children prior to the Industrial Revolution.
With ever-accumulating physical evidence from anthropological studies, combined with advances in the newly emerging scientific disciplines of epigenetics and evolutionary medicine, it can be stated with a reasonable degree of scientific certainty that malocclusion is not primarily a genetically determined disease entity. Rather, malocclusion is better described as a WD that is primarily mediated through a gene–environment interaction that follows a fairly predictable pattern of pathological progression: initially, most WDs are preventable so long as genetically predisposed individuals are identified before early phenotypic expression of the disease is obvious, and where feasible, are allowed to thrive in a nurturing environment; next, WDs can be reversible, but only in the very early stages of disease expression, and only when the precipitating environmental pressures (e.g., unhealthy eating, sleep-disordered breathing) have been eliminated; subsequently, in cases where a WD has advanced beyond reversibility, it can still be treated with accurate diagnosis and appropriate therapeutic measures (e.g., dietary changes, pharmaceuticals) if the disease state is not too far advanced; and finally, advanced end-stage WDs can be fatal if not accurately identified, reversed and/or appropriately controlled.
While a cause-and-effect relationship between malocclusion and the pathophysiology of SDB/OSA is not yet proven,18 a relationship does indeed appear to exist between the two disease entities. Similar to what is now understood about why diabetes and periodontal disease often coexist in the same host,19 the underlying mechanism connecting SDB/OSA and malocclusion is more likely to be a bidirectional one rather than a unilateral cause-and-effect relationship. Simply stated, measures aimed at preventing the initiation and early progression of one disease entity will aid in preventing the initiation and early progression of the other. Given that many dentofacial physical risk indicators for malocclusion might also identify increased risk for SDB/OSA, it seems fairly obvious that measures aimed at prevention, reversal and/or adequate treatment of malocclusion might also help preclude the negative health outcomes that are often associated with SDB/OSA.
Pediatric Oral Health and Sleep
Dentists who treat children are uniquely positioned to identify patients who might be at increased risk for SDB/OSA. Due in part to the successful implementation of the American Academy of Pediatrics and American Academy of Pediatric Dentistry’s (AAPD) joint effort to assure that all children establish a dental home by the age of 1 year,20 pediatric dentists now have a higher frequency of patient encounters than do most other allied health professionals. Additionally, postgraduate specialty training programs in pediatric dentistry and orthodontics purposefully prepare clinicians for identifying patients with interferences to normal dentofacial development, including children with special healthcare needs who might be at even higher risk for developing OSA, such as patients diagnosed with Down syndrome, sickle cell anemia, and Pierre-Robin sequence.
Surgical Versus Non-Surgical Treatment Options for Pediatric SDB/OSA
It is well established that surgical removal of the tonsils and/or adenoids is the most common treatment for pediatric OSA. For extremely severe cases of OSA for which adenotonsillar surgery might not be indicated as the best treatment, maxillomandibular advancement surgery (MMA) and/or tracheostomy placement are on occasion considered as better surgical options. According to a recently published guidelines paper by the American Academy of Otolaryngology,21 craniofacial abnormalities of the maxilla and mandible are definite indications for recommending a PSG sleep study prior to T & A surgery.
Maxillary constriction (MC) is a common craniofacial abnormality that plays an important role in the bi-directional relationship between malocclusion and OSA;22 MC is typically characterized by narrow/deep-vaulted palate (Figure 34-1), tapered dental arches and retro-position of the mid face relative to the anterior cranial base. Per the various comorbidities associated with various surgical interventions, wherever feasible, collaborative efforts aimed at preventing and treating pediatric OSA non-surgically should be given the highest consideration.
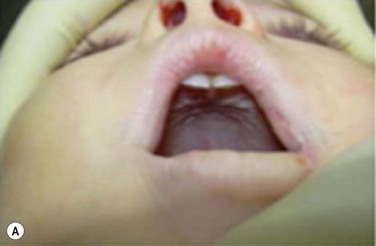
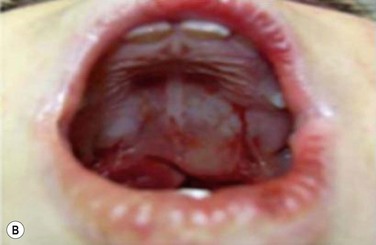
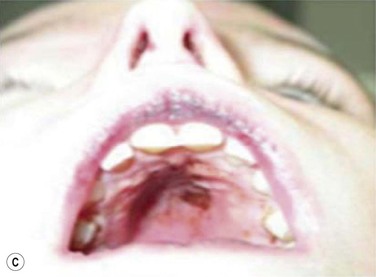
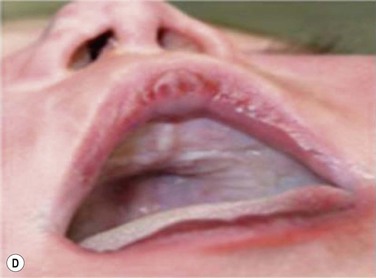
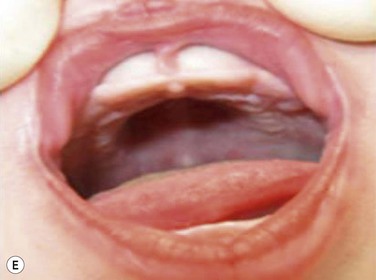
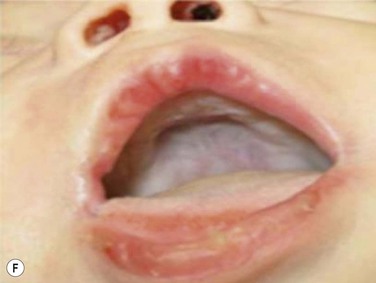
Figure 34-1 Montage of abnormal high and narrow hard palate.
Note that all children present a visually recognizable abnormal high and narrow hard palate which is related to the development of the naso-maxillary complex during embryonic development considering age of children. On the first row on the left, on the second row in both cases, and on the third row from the top on the right, note the abnormal noses presented by the patients. The asymmetry of the nostril may not be obvious at first investigation; using photographs taken below the nose may help performing better analyses. Asymmetrical opening is often associated with asymmetrical septum and change in nasal resistance. When associated with high palatal vault, they indicate presence of a higher upper airway resistance and greater risk of abnormal breathing during sleep with addition of infectious or inflammatory reaction. From Rambaud C, Guilleminault C., Death, nasomaxillary complex, and sleep in young children. Eur J Pediatr. 2012 Sep;171(9):1349–58, with permission of Springer-Verlag 2012.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


