Sepsis Neonatorum
Patrick G. Gallagher
Robert S. Baltimore
Sepsis neonatorum refers to a constellation of clinical and laboratory findings associated with invasive infection during the first 30 days of life. Traditionally, the neonatal sepsis syndrome has been associated with bacteremia, but it may be caused by a variety of pathogens, including bacteria, viruses, and fungi. Neonatal sepsis is an important cause of morbidity and mortality, with case-fatality rates of 15% to 30% even with ideal management. It is important to be familiar with the epidemiology, pathogenesis, etiologic agents, and manifestations of neonatal sepsis so that affected infants receive prompt evaluation and treatment.
EPIDEMIOLOGY
The rate of neonatal sepsis in the United States ranges from 1 to 8 per 1,000 live births, with an average of 2 per 1,000 live births. This rate varies based on socioeconomic factors, availability and utilization of prenatal care, and perinatal risk factors. Worldwide, this rate ranges from 1 to 10 per 1,000 live births, with higher rates in developing countries. Low birth weight and male gender are associated with higher rates of neonatal sepsis. Sepsis rates in preterm infants range from 20 to 30 per 1,000 live births.
Neonatal sepsis usually is classified according to time and mode of onset. Congenital infection is acquired in utero by transplacental or ascending transmission, with onset before birth. Early-onset infection is acquired by transplacental, ascending, or intrapartum transmission in the perinatal period, shortly before or during the process of birth. Late-onset infection is acquired by horizontal transmission, typically in the hospital, at home, or in the community. The appropriate time for dividing early from late-onset infection is not clear, with opinions ranging from 2 to 7 days of age. Approximately 75% of early-onset group B streptococcal (GBS) infections are symptomatic in the first 24 hours of life, and 80% to 90% are symptomatic by 48 hours of age.
Congenital Infection
The major risk factor for congenital infection is maternal infection (Box 71.1). This is typically a primary maternal infection with a pathogen such as syphilis or human immunodeficiency virus (HIV). Prolonged, premature rupture of membranes also is a risk factor.
Early-Onset Infections
Our knowledge of the epidemiology of perinatally acquired bacterial infections is based on extensive studies of GBS, and to a lesser extent, Escherichia coli. The primary risk factor for early-onset GBS disease is the asymptomatic colonization
of the maternal gastrointestinal or genitourinary tracts. GBS colonizes 5% to 40% of pregnant women, with variability in colonization rates attributed to differences in GBS culture techniques and demographic factors. Higher colonization rates are found in African American women and women with diabetes. Lower colonization rates are found in Asian American and Mexican American women, multiparous women, and sexually inexperienced women. GBS colonization throughout pregnancy is inconsistent, even after antibiotic treatment. Mothers not colonized with GBS early in pregnancy may be positive at delivery, and GBS carriers identified early in pregnancy may not be carriers at delivery.
of the maternal gastrointestinal or genitourinary tracts. GBS colonizes 5% to 40% of pregnant women, with variability in colonization rates attributed to differences in GBS culture techniques and demographic factors. Higher colonization rates are found in African American women and women with diabetes. Lower colonization rates are found in Asian American and Mexican American women, multiparous women, and sexually inexperienced women. GBS colonization throughout pregnancy is inconsistent, even after antibiotic treatment. Mothers not colonized with GBS early in pregnancy may be positive at delivery, and GBS carriers identified early in pregnancy may not be carriers at delivery.
BOX 71.1 Major Risk Factors for Neonatal Sepsis*
Congenital infection
Maternal infection, usually primary infection
Prolonged, premature rupture of membranes
Early-onset infection
Maternal infection, usually primary infection
Prolonged, premature rupture of membranes
Prematurity
Septic or traumatic delivery
Fetal anoxia
Male sex
Maternal infection (especially urogenital)
Maternal poverty, poor/no prenatal care, preeclampsia, maternal cardiac disease
Late-onset infection
Extreme prematurity
Bronchopulmonary dysplasia
Complex congenital malformations
Short bowel syndrome
Previous broad spectrum antibiotic therapy
Intravascular catheters
Endotracheal intubation
Assisted ventilation
Surgery (including necrotizing enterocolitis)
Contact with hands of personnel colonized with pathogens
Contact with contaminated equipment
Footnote
*Reproduced with permission from
Baltimore RS. Neonatal sepsis: epidemiology and management. Paediatr Drugs 2003;5:723.
The rate of GBS transmission from colonized mothers to their infants is approximately 40% to 70%. Colonization occurs by transplacental transmission in the presence of maternal bacteremia, ascension from the vagina and cervix through microscopic defects in the amniotic membranes, or through ruptured membranes, surface contamination during passage through the birth canal, and postnatal acquisition in the hospital, at home, or in the community. Despite the high rate of neonatal colonization, only 1% to 2% of colonized infants develop early-onset GBS disease.
The risk factors for early-onset GBS infection (see Box 71.1) include the following: prematurity of less than 37 weeks’ gestation; chorioamnionitis; premature rupture of membranes; prolonged rupture of membranes of more than 18 hours, with the risk of sepsis increasing with the duration of rupture prior to delivery; maternal intrapartum temperature of higher than 38°C; sustained fetal tachycardia; and prior delivery of an infant with GBS disease. Similar associations have been observed with other neonatal pathogens. Black race is a risk factor for both early and late-onset GBS disease. Other factors associated with early-onset GBS sepsis include colonization with a virulent GBS strain, deficient maternal GBS type-specific capsular antibody, maternal colonization at multiple sites, and heavy maternal colonization. Detection of GBS bacteriuria during pregnancy may be a means of identifying the heavily colonized woman whose infant is at increased risk of developing infection. Heavy maternal colonization has been associated with preterm labor, chorioamnionitis, and fetal demise. The twin of an infant infected with GBS is at increased risk, most likely as a result of genetic susceptibility factors, heavy maternal colonization, and/or exposure to virulent strains common for both infants. Maternal diabetes mellitus as a risk factor for neonatal infection is controversial.
Compared to full-term infants, premature infants have a higher risk of neonatal sepsis if the mother has amnionitis or a peripartum infection. Other maternal factors, such as poor prenatal care, low socioeconomic status, and heart disease, are risk factors for both neonatal sepsis and premature birth.
Late-Onset Infections
The risk factors and modes of transmission for late-onset infection in nonhospitalized infants are not well understood. Vertical transmission is responsible for only about 40% to 50% of late-onset GBS disease. The remaining cases are attributed to exposure in the hospital, at home, or in the community.
The epidemiology of late-onset disease in hospitalized infants (see Box 71.1) is discussed in Chapter 88, Nosocomial Infection in the Newborn.
ETIOLOGY
Congenital Infection
Toxoplasma, Treponema pallidum, cytomegalovirus (CMV), and HIV are the organisms most frequently associated with congenital infection, usually during a primary maternal infection. Previously, rubella was a frequent cause of congenital infection, but in the United States, congenital rubella has been virtually eliminated by rubella vaccination. Other causes of congenital infection are the same as early-onset infection.
Early-Onset Infection
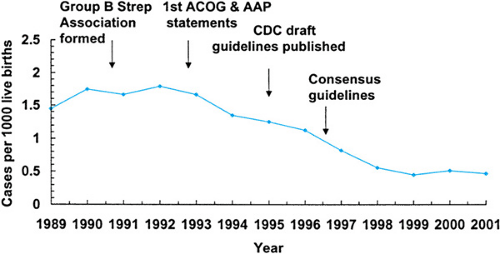
Prior to the 1970s, staphylococci and gram-negative rod species were the predominant etiologic agents of neonatal sepsis. From the 1970s, persisting into the 1990s, GBS was the predominant pathogen causing early-onset infection in most U.S. nurseries (Table 71.1). Since the institution of intrapartum antibiotic prophylaxis (IAP) for the prevention of GBS disease, the rate of early-onset group B Streptococcal infections has fallen dramatically from 1.7 per 1,000 live births in 1993 to 0.6 per 1,000 live births in 2001 (Fig. 71.1). The CDC has predicted that an 80% drop is possible. Paralleling the decrease in early-onset GBS infection is a relative or absolute increase in early-onset disease due to E. coli, in both term and preterm neonates. These two pathogens currently are the major causes of early-onset disease. Other pathogens associated with early-onset sepsis include Klebsiella pneumoniae and other enteric gram-negative bacilli, Enterococcus species, and Listeria monocytogenes. Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae (predominantly nontypeable),
and groups A, C, and G streptococci are respiratory tract pathogens that occasionally colonize the maternal genital tract and cause early-onset neonatal infection.
and groups A, C, and G streptococci are respiratory tract pathogens that occasionally colonize the maternal genital tract and cause early-onset neonatal infection.
TABLE 71.1. MICROORGANISMS CAUSING NEONATAL SEPSIS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Late-Onset Infection
In the 1980s and 1990s, improvements in neonatal intensive care, particularly for the preterm infant, were paralleled by an increase in nosocomial infection by opportunistic organisms such as coagulase-negative staphylococci and Candida species in late-onset sepsis. Other organisms associated with nosocomial late-onset disease include Staphylococcus aureus (methicillin-sensitive and organisms such as coagulase-negative staphylococci and Candida species in late-onset sepsis. Other organisms associated with nosocomial late-onset disease include Staphylococcus aureus (methicillin-sensitive and -resistant), Enterobacter, Serratia, and Pseudomonas species, and Enterococcus (vancomycin-sensitive and -resistant) species Nosocomial outbreaks with these species may occur.
In nonhospitalized infants, the etiologic agents of late-onset disease comprise the organisms causing early-onset disease, particularly GBS and E. coli, gram-positive organisms including S. pneumoniae, S. aureus, and Enterococcal species, and gram-negative enteric organisms including Klebsiella species and Salmonella species. Since the institution of IAP for prevention of GBS disease, unlike the decrease seen in early-onset disease, there has been no change in the rate of late-onset GBS disease. It will be important to monitor whether IAP will influence the etiology of late-onset sepsis.
The significance of anaerobes isolated from blood cultures of neonates remains controversial. Most anaerobic bacteremias are self-limited in the absence of a focal infection. However, Bacteroides and Clostridium species may be associated with significant disease, especially when peritonitis, fasciitis, or meningitis is present.
PATHOGENESIS
Risk factors for the development of neonatal sepsis include maternal and obstetric factors, virulence factors of the causative organism, and neonatal factors. Factors specific to nosocomial, late-onset infections are reviewed in Chapter 88, Nosocomial Infection in the Newborn.
Maternal and Obstetric Factors
Prior to birth, the placenta and its membranes serve as a barrier to fetal infection, maintaining the fetus in a sterile environment. Acquisition of infection before birth via the maternal bloodstream is rare because of the effectiveness of the placental barrier. Obstetric procedures such as amniocentesis, percutaneous umbilical blood sampling, transcervical chorionic villus sampling, or cervical cerclage may introduce skin or vaginal microorganisms into the sterile uterine cavity, leading to amnionitis and secondary fetal infection.
A common mechanism of fetal colonization and infection is via ascending infection. Organisms from the mother’s cervix and vagina invade the amniotic fluid and uterine cavity through microscopic defects or overtly ruptured amniotic membranes. Ingestion and inhalation of these organisms in the amniotic fluid leads to contamination of the respiratory and gastrointestinal tracts, and may lead to bronchopneumonia before birth. Pathogens may adhere to epithelial cells on mucosal surfaces, and then invade the infant’s bloodstream. Common entry sites are the conjunctiva, nasopharynx, umbilical cord, and traumatized integument. Delayed clearance from the bloodstream allows pathogens to multiply and cause either disseminated or focal disease. Signs of infection after ascending infection may be present at delivery, within hours of birth, or, less commonly, within several days.
Another common source of neonatal colonization and infection occurs during passage through the birth canal. Contamination with organisms of the mother’s cervical, vaginal, or fecal flora occurs by surface contact or aspiration during the process of delivery. How and why colonization, which is common, leads to infection, which is not, is poorly understood.
Virulence Factors
A major bacterial virulence factor is the surface polysaccharide capsule. This capsule determines the specific bacterial serotype and provides many bacteria with the ability to evade killing by providing mechanisms to escape opsonophagocytic killing. The sialic acid–rich polysaccharide surface capsule of GBS has been studied extensively, and all major serotypes of GBS (Ia, Ib, Ia/c, II-VIII) have caused early-onset disease. The GBS capsule acts as a bloodstream survival factor by inhibiting the alternate complement pathway, protecting GBS from opsonization by C3 and subsequent neutrophil killing. Maternal levels of antibodies to type-specific capsular polysaccharides correlate well with protection from GBS.
Other GBS virulence factors include an extracytoplasmic penicillin-binding protein, which promotes resistance to neutrophil killing independent of the polysaccharide capsule; a surface protease that promotes virulence, resistance to opsonophagocytosis, and cleavage of human fibrinogen; and a surface-localized streptococcal beta-protein that resists opsonophagocytosis by down-regulating complement activation.
Bacterial virulence factors that influence the pathogenesis of most types of neonatal meningitis are the surface polysaccharide capsule and/or the production of cytolytic exotoxins. Two virulence factors for GBS meningitis are well characterized: the type III polysaccharide capsule and the beta-hemolysin/cytolysin, a cytolytic exotoxin. The type III GBS polysaccharide capsule is associated with approximately 90% of cases of GBS neonatal meningitis. Its invasiveness is probably due, in part, to poor maternal antibody response to the type III polysaccharide capsule. Similarly, the K1 serotype of E. coli and the IVb serotype of L. monocytogenes have been associated with neonatal meningitis. Beta-hemolysin/cytolysin is a pore-forming, membrane-associated cytotoxin that contributes to disease virulence and progression via several mechanisms, including the activation of neutrophil signaling pathways in brain endothelium.
Neonatal Factors
The neonate is a compromised host at risk for invasive infection because of developmental defects in cellular, humoral, and phagocytic immunity. Complement levels are decreased. IgG, an important component of the immune response, is acquired from the mother during the third trimester, resulting in a concentration in the blood of the newborn slightly higher than that of the mother. The specificities of the antibodies and the protection afforded are similar to the mother’s. If the infant is premature, the amount of IgG acquired from the mother is decreased, and the neonate is susceptible to those pathogens. In some cases, additional genetically predetermined immune defects or other factors predisposing to infection may be present.
Further impairment to immunity occurs under conditions of prematurity, hypoxia, acidosis, and other metabolic derangements associated with bacterial infection. Infants with galactosemia are particularly susceptible to infection with gram-negative rods, particularly E. coli.
Finally, the neonate, particularly the preterm infant, has poor skin and mucosal barriers to infection. These anatomic barriers may be further compromised by trauma before, during, or after delivery, such as those caused by fetal scalp electrode placement, fetal scalp blood sampling, abrasions during delivery, injury from obstetric forceps, umbilical catheterization, and the like.
CLINICAL MANIFESTATIONS AND COMPLICATIONS
The clinical signs of neonatal sepsis are nonspecific (Box 71.2). They may be associated with bacterial, viral, or fungal
infection, or with noninfectious disorders including metabolic disorders, intracranial hemorrhage, congenital heart disease, or perinatal asphyxia (Table 71.2). Presentation is variable, from subtle findings such as feeding intolerance, jaundice, and temperature instability to acute onset respiratory failure and septic shock. When pneumonia is present, signs of respiratory distress such as tachypnea, grunting, nasal flaring, retractions, and cyanosis may occur. The clinical course is unpredictable and may be rapidly progressive. The presence of these signs should lead to consideration of a diagnostic sepsis evaluation and prompt initiation of empiric antimicrobial therapy.
infection, or with noninfectious disorders including metabolic disorders, intracranial hemorrhage, congenital heart disease, or perinatal asphyxia (Table 71.2). Presentation is variable, from subtle findings such as feeding intolerance, jaundice, and temperature instability to acute onset respiratory failure and septic shock. When pneumonia is present, signs of respiratory distress such as tachypnea, grunting, nasal flaring, retractions, and cyanosis may occur. The clinical course is unpredictable and may be rapidly progressive. The presence of these signs should lead to consideration of a diagnostic sepsis evaluation and prompt initiation of empiric antimicrobial therapy.
BOX 71.2 Nonspecific Signs of Sepsis
Lethargy
Apnea
Temperature instability
Tachypnea
Tachycardia
Pallor, poor perfusion
Respiratory distress
Feeding intolerance
Vomiting
Abdominal distention
Diarrhea
Jaundice
Skin rash, petechiae
Hypotension
Irritability
High-pitched cry
Weak suck
Seizures
Bulging or full fontanel
TABLE 71.2. DIFFERENTIAL DIAGNOSIS OF THE SEPTIC-APPEARING INFANT* | ||
|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree