Selected Topics in Emergency Medicine
Paula J. Schweich
Often, visits to the emergency department (ED) are stressful for children and parents. The staff members are strangers to the children, the environment may appear chaotic, and the rooms can be confining and sterile. If the ED is busy, necessitating a wait, children can become tired and restless, and the parents can become impatient. The ED staff should make every effort to ensure that children and parents are as comfortable and calm as possible.
HISTORY TAKING
Chief Complaint
If emergent treatment is needed, a brief history relevant to the problem is obtained so that appropriate therapy can be instituted. After emergent therapy is administered, a more detailed history can be obtained. This detailed history includes a description of the complaint through the length of time of illness, behavior of the affected child during the illness (e.g., interaction and feeding), and associated signs and symptoms. A full review of systems may reveal other important parts of the history.
MEDICAL HISTORY
A short medical history may reveal problems that were not discussed during the initial history. This history should include previous illnesses, including similar symptoms; hospitalizations; allergies to medications; medications past and present; surgeries; and status of immunizations. This information should be included in all ED records.
Physical Examination
The physical examination for all children begins with an assessment of toxicity. Particularly in young children, physicians should have a good idea of how ill an affected child appears before touching the child. Observation of the child should begin during the history-taking portion of the visit. The examination techniques depend on the child’s age. In most children, the quiet and nonintrusive parts of the examination are performed first, such as assessment of heart and lungs, and the more threatening parts are examined last, such as assessment of ears and throat. The examination should focus on the system involved
with the chief complaint, but a complete physical examination is necessary.
with the chief complaint, but a complete physical examination is necessary.
Infants
Assessing the level of toxicity in small infants can be difficult. The history may include poor feeding, lethargy, or excessive sleepiness, and the seriousness of the complaints must be assessed by the physical examination. After observing an affected child in the parent’s arms, the physician might hold the infant on his or her lap; this is helpful in assessing the degree of interaction and vigor. If the complaint involves feeding, infant feeding should be observed. During this time, the child’s state (e.g., fussiness or irritability) is assessed, and the tone of the extremities is noted. With the baby either on the physician’s lap or on the table, the anterior fontanel, heart, and lungs can be assessed, and abdominal examination can proceed. The results of the remainder of the examination, including skin and genitalia, can be noted as the examination proceeds.
Toddlers
Examining a toddler can be a challenge. Often, toddlers are restless and hungry in the ED, and they may react to a physician with stranger anxiety. Initial observation should note whether the child is fussy but calms with a parent, or is irritable and inconsolable. The physician may find that sitting on a stool with wheels is helpful. This approach allows the physician to begin the history taking at a distance from the toddler and to move closer during the discussion. At the end of the history taking, the child might be engaged with an interesting object, such as a beeper or stethoscope toy. If possible, the entire examination should be performed with the toddler in a parent’s lap, where the child will feel most comfortable.
In toddlers, starting with an examination of the heart and lungs is crucial. After that phase, the abdomen should be palpated with the child sitting or lying in the parent’s lap. This may be the only calm abdominal examination possible. In the presence of concern about the abdomen, further examination is attempted on the table. Examination of the extremities also can be performed on the parent’s lap. For an extremity complaint, the parent can palpate and move the child’s extremities first, starting with the uninvolved side. This technique should give a sense of whether the child is reacting to pain in the extremity. After the parent examines the limbs, the physician should attempt to examine the extremities in the same order. As in younger infants, the ears and throat should be examined last. This phase also can be performed on the parent’s lap, with the child facing forward. The parent places one arm over the child’s arms and the other arm over the child’s head, firmly holding the head to one side. The throat can be examined with the head held straight forward.
Preschoolers
Because preschool-aged children are developing language skills, they may be able to describe what is bothering them, and the physician can explain calmly what is happening during the examination. The child may be eager to participate with the examination (e.g., holding the stethoscope on the chest). During parts of the examination, the child can be distracted with stories or explanations. For example, in examining the abdomen, if the examiner claims to be trying to feel for what the child ate during the last meal, the child is likely to lie very still and cooperate. Children at this age also feel fear and are imaginative; therefore, it is important to explain often that what is being done will not hurt. However, if a painful procedure is to be performed, such as drawing blood, the physician must be honest and explain what will happen.
School-Aged Children
Examining school-aged children is much easier than examining younger children, because these older children have well-developed language skills, a capacity to reason, and often a basic understanding of why they are in the ED. It is important to tell school-aged children why any treatment procedures are necessary.
Adolescents
Adolescents should be treated as adults with regard to explanations and privacy. Their medical history may be started with a parent in the room, but it is important to examine adolescents in the absence of the parent. More sensitive questions, such as those about sexual activity and drug use, can be asked during this phase of the examination. Also, it is important to address any fears that adolescents may have in regard to their illness.
CARDIOPULMONARY RESUSCITATION IN CHILDREN
Most pediatric cardiopulmonary arrests occur in young, previously healthy children. Every attempt should be made to identify the cause of the arrest, because special considerations may affect treatment. Noncardiac causes predominate; trauma is the leading cause of cardiopulmonary arrest in children 1 to 14 years of age. The most common causes are listed in Box 116.1.
In most patients with out-of-hospital arrest, a progression occurs from hypoxia and hypercarbia, to respiratory arrest and bradycardia, to asystolic cardiac arrest. In the case of acutely ill or injured children, the rescuer may be able to prevent respiratory and circulatory arrest with the correction of hypoxia alone. Because many causes of arrest in children are preventable and because elapsed time until initial resuscitation is such an important factor in survival, special efforts should be made in prevention and prehospital care.
Children who have delayed resuscitation or present in asystole have a poor prognosis, because hypoxemia already will have caused extensive damage to the brain and other vital organs. Survival is more likely if cardiopulmonary resuscitation (CPR) is started immediately, if only respiratory arrest is
present, if the arrest is witnessed in the hospital, if the condition is extreme bradycardia rather than asystole, or if only oxygen is necessary.
present, if the arrest is witnessed in the hospital, if the condition is extreme bradycardia rather than asystole, or if only oxygen is necessary.
BOX 116.1 Common Causes of Cardiac Arrest
|
Basic Life Support
The goals of life support are to optimize cardiac output and to sustain tissue oxygen delivery; most important are the metabolic demands of the myocardium and brain. Because respiratory failure is the most common cause of cardiac arrest in children, basic life support should begin immediately after discovery of the arrest victim. If there is only one rescuer, the cause of the emergency is unknown, and the child is 8 years old or younger, emergency medical services (EMS) should be notified after CPR has been performed for 1 minute (“phone Fast”). In older children, activate EMS first, and then start CPR (“phone First”).
When initially approaching the victim, the rescuer should note whether there is any movement, crying or breathing, and color. The first priority is to assess the adequacy of airway, breathing, and circulation. For children who are accident victims and may have a neck injury, the neck should be stabilized with axial traction until a Philadelphia collar can be applied. Gently shaking and calling to the victim help determine degree of response or presence of respiratory difficulty. Affected conscious children should be allowed to position their airway; children automatically assume the best position. Unconscious children should be positioned on a firm surface. When affected children are moved or turned, the head, neck, and torso should be moved as a single unit. If a neck injury is not suspected, the examiner can place one hand on the forehead to tilt the head back to a neutral position. Overextension of the neck should be avoided, because it obstructs the trachea. The fingers of the free hand are placed under the lower jaw at the chin to lift the chin off the airway. For further movement of the jaw, the rescuer’s hands are placed on both sides of the victim’s head, allowing the palms to tilt the head back. Two or three fingers are placed at each mandibular angle to lift the jaw upward (i.e., jaw thrust). If a neck injury is suspected, the jaw thrust can be used without a head tilt.
After the airway is opened, the rescuer simultaneously should evaluate the chest wall and abdomen for movement, listen over the mouth and nose, and feel with the cheek for air flow. If a child is not breathing, and the airway is in the correct position, the rescuer should deliver two effective slow breaths immediately. The rescuer’s mouth can cover the mouth and nose of an infant, or the nose (in older children) can be pinched closed for mouth-to-mouth breathing. In successful mouth-to-mouth breathing, chest movement is visible. If it is unsuccessful, the rescuer should try to reposition the airway and reattempt ventilation. If still unsuccessful, the rescuer should check for a foreign body in the mouth or pharynx.
A child’s airway is obstructed easily by aspiration of liquids and small objects (e.g., mucus, blood, vomitus, the tongue, hard candies, popcorn, nuts, removable parts of toys). Initially, an affected child coughs and gags but, if the obstruction is complete and the airway cannot be cleared, the child loses consciousness. The rescuer should attempt to relieve the obstruction only if the child’s cough is weak and ineffective and respiratory difficulty is increasing.
If aspiration is witnessed or strongly suspected, or if an unconscious victim has airway obstruction that cannot be relieved by head-tilting and jaw-thrust maneuvers, the rescuer should attempt to remove the object manually—but only if it is visible on careful inspection. Blind finger sweeps may push a foreign body farther into the airway and should be avoided.
The Heimlich maneuver, a subdiaphragmatic abdominal thrust, produces an artificial cough and is considered safe for children older than 1 year of age. With the child supine, the heel of the rescuer’s hand is placed in the midline between the umbilicus and rib cage and is pushed rapidly inward and upward. This maneuver can be repeated five times. It is not performed in a child younger than 1 year of age because of concern for intraabdominal injury. For children younger than 1 year of age, alternating back blows (five blows between the scapulae) and chest thrusts (five compressions, as in CPR) are recommended.
After the two rescue breaths are delivered, the victim is assessed for signs of circulation. Signs of circulation include adequate breathing, coughing, and movement. Health care providers should also perform a pulse check. The brachial pulse is checked in an infant; the carotid pulse in a child. If there are uncertain or no signs of circulation, compressions are started immediately. In small infants, the rescuer can encircle the infant’s chest with both hands, support the back with the fingers, and compress the lower half of the sternum with both thumbs. As in adults, children’s hearts lie under the lower third of the sternum, and compressions should be performed over this area. The compression phase should be 50% of the cycle. Chest compressions should produce palpable pulses in a central artery.
Chest compressions and ventilation are coordinated at the rate of five compressions to one ventilation (5:1) for infants and children up to 8 years of age. This high ratio of ventilations to compressions is to correct the hypoxia and hypercarbia that is the most common cause of pediatric cardiorespiratory arrest. A ratio of 15 compressions to 2 ventilations (15:2) is recommended for children 8 years of age and older. The compressions are delayed for ventilation. Table 116.1 outlines breathing and circulation requirements for basic life support. Patients should be reassessed 1 minute after resuscitation begins and again every few minutes.
TABLE 116.1. BASIC LIFE SUPPORT | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 116.2. RESUSCITATION BAGS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Advanced Cardiac Life Support
When children arrive in the ED, basic life support should be in progress. An initial assessment by the emergency physician includes level of consciousness, spontaneous respiratory effort, pulse, blood pressure, cardiac rhythm, temperature, perfusion, and pupillary responses. In advanced life support, as in basic life support, an assessment of airway, breathing, and circulation should begin the rescue.
Airway and Breathing
Children have a higher oxygen demand than adults because they have a higher metabolic rate. Therefore, with apnea or poor ventilation, hypoxemia develops rapidly. Mouth-to-mouth resuscitation provides at most 17% of the fraction of inspired oxygen in advanced cardiac life support. Humidified 100% oxygen should be administered to affected patients immediately on arrival at the hospital (or sooner if possible). The bag, valve, and mask setup (later to be converted to bag, valve, and endotracheal tube) should deliver 100% oxygen and should be equipped with a manometer and pressure-relief valve. Table 116.2 lists the differences between the two types of resuscitation bags. Ventilation bags used for resuscitation should be self-inflating. While equipment for intubation is being prepared (Box 116.2), the bag and valve should be fitted to a clear plastic mask that allows for an airtight seal against the child’s face and for a small rebreathing volume. The soft circular masks seal well, and clear masks allow the physician to observe the color of the child and to see any vomitus. An oropharyngeal airway in an unconscious child or a nasopharyngeal airway in a conscious child helps keep the tongue forward during mask ventilation. A shortened tracheal tube can be used as a nasopharyngeal airway. Placing the airway next to the face allows the physician to determine the size of the oropharyngeal airway. With the flange at the central incisors, the tip of the airway should be at the angle of the mandible. The length of a nasopharyngeal airway is determined by the distance from the tip of the nose to the tragus of the ear.
Intubation should be performed as soon as possible if the patient continues without spontaneous respirations or needs prolonged ventilation. Intubation provides better ventilation, higher oxygen concentration delivery, protection against aspiration, ability to suction secretions from the airways, and the ability to give positive end-expiratory pressure to the patient.
BOX 116.2 Equipment for Intubation
Oxygen source
Face masks and resuscitation bag
Cardiorespiratory monitor with pulse oximeter
Large-bore suction catheter and suction machine
Tracheal tubes and stylet
Laryngoscope blade and handle
Tape to secure tube
Exhaled CO2 detector
The pediatric airway is more flexible than is the adult airway, the tongue is relatively larger, and an anatomic narrowing exists at the level of the cricoid cartilage, precluding the necessity of using a cuffed endotracheal tube in children younger than 8 to 10 years of age. A cuffed tube with a low-pressure cuff is used for older children. An endotracheal tube of appropriate size is chosen, and one size larger and smaller should be available. If a stylet is used, its tip should be 1 to 2 cm proximal to the end of the endotracheal tube. The formula for determining endotracheal tube sizes is:
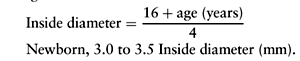
The depth of insertion from the distal end of the tube to the lip can be estimated by multiplying the internal diameter of the tube by 3. The Broselow tape allows for a length-based determination of endotracheal tube size and drug dosages for cardiac arrest and seizure. Any area that cares for ill children should be equipped with this piece of equipment.
Before intubation, the patient should be preventilated with 100% oxygen by means of a bag, valve, and mask setup, and suction should be easily accessible. During use of the bag, valve, and mask, and during intubation, an assistant should apply pressure to the cricoid cartilage using the thumb and forefinger, to push the esophagus up against the cervical spine. This decreases inflation of the stomach and the risk of aspiration. The mouth and pharynx are suctioned immediately before intubation. The axes of the mouth, pharynx, and trachea must be aligned to directly visualize the glottis. The chin is lifted into a sniffing position. The tip of a straight blade is placed under the epiglottis and is lifted, or a curved blade is placed into the vallecula, the glottic opening is visualized, and the endotracheal tube is placed between the cords. If ventilation is interrupted for more than 20 seconds or the heart rate decreases to less than 60 beats per minute, mask ventilation should be resumed before another intubation attempt. As soon as the endotracheal tube has been placed, the bag and valve are attached for hand ventilation until a ventilator is available. After the clinician checks for symmetric chest movement and bilateral chest sounds, the tube is secured at the mouth, and the position is confirmed by chest radiography. A colorimetric end-tidal carbon dioxide detector may be used as an adjunct to confirm tube placement; however, a negative test (lack of color change) may not indicate esophageal intubation but rather airway obstruction, or poor or absent pulmonary blood flow. The use of an esophageal detector device that confirms endotracheal intubation by the aspiration of air from a correctly placed endotracheal tube also may be useful in older children, but further study is needed in the pediatric age group.
Pulse oximetry is an excellent noninvasive technique for continuously monitoring arterial oxygen saturation before, during, and after intubation. It does not, however, measure the effectiveness of ventilation. Arterial blood gas analysis is still the standard best way to assess the effectiveness of oxygenation, ventilation, and response to therapy.
Box 116.3 lists the possible causes of sudden deterioration in an intubated patient. Tracheal tube position is assessed by observation of chest wall movement during manual ventilation, auscultation, and checking tube placement with the laryngoscope. If tube obstruction is suspected, saline is instilled into the tube and suctioned with a catheter. If the tube in occluded, it must be replaced.
BOX 116.3 Deterioration in an Intubated Patient: “DOPE”
Displacement of the tube from the trachea
Obstruction of the tube
Pneumothorax
Equipment failure
Footnote
Adapted with permission from PALS Provider Manual, AHA.
Tension pneumothorax is confirmed by decreased breath sounds on the affected side and a radiograph confirming collapsed lung and deviation of the trachea and mediastinal structures away from the affected side. If this is suspected, immediate needle decompression must be performed.
Equipment also should be checked in the presence of patient deterioration. It is essential that the oxygen circuit is uninterrupted and that no leaks are present. The also monitors should be checked.
Circulation
While an airway is being secured, other members of the resuscitation team should be obtaining intravenous access and monitoring pulse and cardiac rhythm. The patient is placed on a hard surface, and chest compressions are performed in the absence of a pulse. Intravenous access is crucial for drug and fluid administration, but achieving it is often difficult in a child with poor circulation. Usually, placing a peripheral intravenous line during an arrest is difficult, and placing a central line in the neck area interferes with resuscitation. The preferred methods for access are femoral vein catheterization, greater saphenous vein cutdown, or intraosseous vascular access. Often, an intraosseous infusion is the easiest and quickest method of access to the circulation and can be used in children of any age. It provides access to the noncollapsible marrow venous plexus, and is a reliable route for administration of drugs, fluids, and blood. The method is outlined in Box 116.4.
The possible causes of the patient’s arrest are considered before the administration of intravenous fluids. If a child with prehospital cardiac arrest of unknown cause fails to respond to initial resuscitation measures, consider an initial bolus (20 mL/kg) of a crystalloid. Patients with acute blood loss, shock, or dehydration may require vigorous volume replacement; those with head trauma or hypernatremic dehydration may be harmed further by overzealous fluid administration. Rapid volume expansion is accomplished best with isotonic crystalloid solutions, such as lactated Ringer’s solution or normal saline, until such colloid as blood, fresh-frozen plasma, or human serum albumin is available.
Drugs
Various drugs are used to increase the heart rate, to correct hypoxemia, to reverse metabolic acidosis, to improve cardiac contractility, or to increase coronary and cerebral perfusion pressure. The goal is to restore spontaneous circulation and to stabilize an affected child’s cardiac rhythm.
BOX 116.4 Intraosseous Infusion
Choose site.
Flat smooth surface of anterior tibia (1–3 cm belowtuberosity). Contralateral tibia.
Distal femur (1–2 cm proximal to superior border ofpatella).
Insert needle.
Use bone marrow needle or 18-gauge spinal needle.
Angle away from joint space.
Use firm twisting motion until decreased resistance.
Check placement.
Remove stylet.
Aspirate marrow if possible.
Flush with saline, even if no aspirate.
Monitor for tissue swelling.
Administer drug.
Use any drug or fluid that can be given intravenously.
Asystole and sinus bradycardia account for 90% of arrhythmias in pediatric patients in arrest. Because primary cardiac disease is a rare cause of cardiac arrest, other ventricular arrhythmias are uncommon. Box 116.5 lists the reversible causes of life-threatening arrhythmias or cardiac arrest.
The preferred route of administration of drugs during resuscitation is intravenous bolus or infusion. In the event of a delay in establishing intravenous access, the lipid-soluble drugs epinephrine, lidocaine, atropine, and naloxone may be given through the endotracheal tube. The tracheal dose of epinephrine is 10 times the intravenous route dose. For endotracheal administration, the drug should be diluted to 5 to 10 mL, pushed in rapidly, and followed by five positive-pressure breaths. All drugs and fluids may be given by intraosseous infusion. Intracardiac injection has serious risks and is not recommended. Figure 116.1 displays protocols for cardiac arrest and arrhythmias. Epinephrine is the key medication used in cardiac arrest. The most important pharmacologic action of epinephrine in cardiac arrest is the alpha-adrenergic–mediated vasoconstriction, which increases aortic diastolic pressure and coronary perfusion pressure. It also increases cardiac automaticity, heart rate, myocardial contractility, and blood pressure. The use of high-dose epinephrine does not improve
outcome, and can worsen post-resuscitation cardiac dysfunction. It is not routinely recommended.
outcome, and can worsen post-resuscitation cardiac dysfunction. It is not routinely recommended.
BOX 116.5 Potentially Reversible Causes of Cardiac Arrest: The 4 H’s and the 4 T’s
Hypoxemia
Hypovolemia
Hypothermia
Hyperkalemia/Hypokalemia
Tamponade (cardiac)
Tension pneumothorax
Toxins/poisons/drugs
Thromboembolism
Footnote
Adapted with permission from AHA Guidelines 2000.
The treatment of sinus bradycardia with cardiovascular compromise (heart rate less than 60 beats per minute with poor systemic perfusion) is similar to the treatment of asystole; in addition, atropine, 0.02 mg/kg (minimum, 0.1 mg; maximum, 1.0 mg), should be considered. Because hypoxemia is the most common cause of bradycardia, it is necessary to ensure adequate oxygenation and ventilation.
Ventricular arrhythmias may occur in patients with congenital heart disease, myocardial disease, chest trauma, or drug ingestions. Ventricular fibrillation and pulseless ventricular tachycardia are initially treated with electrical defibrillation, which causes sudden depolarization of the myocardium, to allow intrinsic cardiac automaticity to resume. If defibrillation is not possible immediately, the physician should try to correct hypoxemia and acidosis while setting up the defibrillator.
Pediatric paddles are available in 4.5-cm and 8.0-cm sizes. The correct paddle size is that which allows the entire paddle surface to contact the chest wall. One is placed to the right of the sternum below the clavicle, and the other is placed on the left anterior axillary line at the level of the nipple (i.e., apex). The electrode–skin interface can be electrode paste or cream; care is taken to apply interface only under the paddles—bridging causes a short circuit. Alcohol pads can cause burns and should never be used as the interface.
The initial dose for defibrillation is 2 J/kg. Subsequent doses are doubled. The procedure must be stopped if the rhythm converts out of ventricular fibrillation. If three shocks do not correct the rhythm, CPR should be resumed with drugs, and the patient should be assessed again for acidosis, hypoxemia, and hypothermia.
If the monitored rhythm is ventricular tachycardia and the patient is symptomatic, the patient undergoes synchronized cardioversion, which is a timed depolarization of myocardial cells and therefore different from defibrillation. The synchronizer circuit must be activated, and the dose is 0.5 to 1 J/kg. If the rhythm is refractory to cardioversion, amiodarone, procainamide, or lidocaine may aid further attempts at cardioversion.
Frequent reassessment, including that of body temperature, is mandatory during any resuscitation.
Parental Presence During Resuscitation
It is common practice in pediatric EDs to have parents present for minor invasive procedures such as venipuncture, intravenous access, urinary catheterization, arterial blood gases, and laceration repair. The vast majority of parents prefer to be present for a procedure on their child; their presence reduces the stress for both the child and the parent(s). The parent may just soothe the child with familiar encouraging words, or in some cases actually help restrain the child for the procedure. Most physicians are comfortable performing even more invasive procedures, such as lumbar puncture, with parents present. Studies show that parental presence does not negatively affect the success of the procedure.
The practice of family member involvement during pediatric resuscitations remains controversial. As with other illnesses and procedures, family members are frequently interested in being present for the resuscitation in order to support their child. It facilitates their understanding of the gravity of the situation and the efforts being made, and the acceptance of the death of their child, if it occurs. Often, the family is present during the pre-hospital resuscitation, but is excluded after arrival at the hospital. It has long been the standard policy in most EDs to exclude family members from the treatment room during the resuscitation of cardiopulmonary arrest and trauma victims. The reluctance of ED staff to permit family involvement may be based on fears that family members will interfere with medical efforts, that observation may reveal apparent weaknesses and failure of medical care, or that the staff may become uneasy and lose concentration and objectivity. It may understandably be more difficult to end the resuscitation efforts with family members present. In addition, many health professionals assume that presence during resuscitation efforts would be an undesirable experience for family members. However, in multiple studies, the responses from relatives who witnessed the resuscitation of their loved one were overwhelmingly positive. The majority of family members who are allowed to be present during resuscitations favor this practice. In fact, in many cases, it helps with the family’s grieving process.
If family members are present for a resuscitation, the team should act sensitively to the family’s presence, the family should be prepared by the staff for what they will see, a staff member, such as a social worker, should be assigned to the family to act as a liaison, and there should be a seat or space for the family member. If the resuscitation is unsuccessful, the family should be allowed time to say good-bye.
When determining whether family members should be present during a resuscitation, the concerns of both the family and the ED personnel should be addressed. Relatives should not be viewed as an added burden to the staff, but as a part of the medical care and process. Research shows that medical providers who have experienced having family members present during resuscitation favor this practice.
AIRWAY OBSTRUCTION
Airway obstruction may result from processes in the upper or lower airway. Upper airway refers to the level above the secondary bronchi, and lower airway refers to peripheral airways, which usually are less than 3 mm in diameter. Usually, lower airway obstruction involves a diffuse distribution of obstruction. The differentiating characteristics are listed in Table 116.3.
Upper airway obstruction interferes primarily with inspiration. As upper airway obstruction progresses, a small increase occurs in respiratory rate, and a large increase occurs in respiratory effort, leading to dyspnea. The increased respiratory effort causes an increased negative intrathoracic pressure, manifested by retractions. Stridor, a low-pitched respiratory sound, results.
TABLE 116.3. AIRWAY OBSTRUCTION | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Lower airway obstruction interferes with the expiration of air from the smaller airways, causing the expiratory phase to be prolonged. The turbulent air flow is heard as wheezing. As the obstruction increases, accessory muscles are used. Wheezing increases as the obstruction progresses, and then decreases as air flow becomes limited.
Foreign-body aspiration, croup, epiglottitis, abscesses, asthma, and bronchiolitis are the more common causes of airway obstruction seen in children.
Foreign-Body Aspiration
Foreign-body aspiration is a significant health hazard in young children, particularly those between 6 months and 3 years of age.
Pathophysiology
Most foreign bodies that children aspirate are small and, rather than lodging in the trachea and causing acute obstruction, pass through the trachea to lodge in a main stem bronchus. These small foreign bodies, usually composed of organic matter, are not immediately life-threatening. Peanuts are the most common objects to be aspirated.
Diagnosis
Frequently, the diagnosis of foreign-body aspiration is missed, because children often present with no known history of aspiration, and only subtle signs and symptoms. The diagnosis requires a high index of suspicion and should be considered in any child with unexplained pulmonary complaints. A history of sudden-onset choking or coughing while eating or playing with small objects is helpful but not always obtainable. With delayed diagnosis, affected children may present with recurrent attacks of wheezing diagnosed as asthma, pneumonia, or bronchiectasis.
In most children, a history of the sudden onset of coughing with acute respiratory distress or subsequent coughing, wheezing, or stridor suggests the diagnosis of foreign-body aspiration. The clinical symptoms depend on the location of the
foreign body (Table 116.4). The most frequent physical findings are wheezing, decreased air movement, and rhonchi, all localized over the lung with the involved airway.
foreign body (Table 116.4). The most frequent physical findings are wheezing, decreased air movement, and rhonchi, all localized over the lung with the involved airway.
TABLE 116.4. FOREIGN-BODY ASPIRATION | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
In children with foreign-body aspiration, the chest radiographs range from diagnostic to totally unremarkable. The appropriate views include inspiratory and expiratory or bilateral decubitus chest radiographs. Persistent air trapping on an expiratory or lateral decubitus radiograph may be found. Persistent atelectasis or infiltration are other common findings. The foreign body itself rarely is opaque and, therefore, rarely is visible on the radiograph. If initial films appear equivocal or normal, and aspiration is still suspected, fluoroscopy should be performed. Often, the difference in chest wall expansion that may occur with foreign-body aspiration is detected by fluoroscopy.
Therapy
If the child presents to the ED with no air movement, the Heimlich maneuver or back blows are administered (see section Cardiopulmonary Resuscitation in Children). Children with asphyxiating foreign-body aspiration require immediate rigid bronchoscopy for foreign-body removal. However, most aspirations are not life-threatening, and a thorough history and physical examination can be obtained.
If suspicion of foreign-body aspiration is supported by physical examination or radiographs, bronchoscopy is the treatment of choice for foreign-body removal. On radiographic evidence of a radiopaque foreign body or on other strong evidence of a foreign-body aspiration, such as unilaterally decreased breath sounds or obstructive emphysema, rigid bronchoscopy should be performed for foreign-body removal. If the evidence for an aspiration is less convincing, flexible bronchoscopy can be performed first to confirm the diagnosis; then, rigid bronchoscopy can be performed if a foreign body is found. Using flexible bronchoscopy first avoids the risks and expense of general anesthesia in those patients without an aspiration. The degree of urgency depends on the location of the foreign body and the degree of respiratory distress.
Croup
Croup, or laryngotracheobronchitis, is a common syndrome involving inflammation or edema of the subglottic area, causing airway obstruction in the larynx, trachea, or bronchi.
Epidemiology
Usually, croup affects children of 6 months to 4 years of age, with the peak incidence at 1 to 2 years of age. Croup accounts for more than 90% of stridor with fever. It occurs all during the year, but the incidence increases in late fall and winter.
Pathophysiology
Most croup is viral, but it also may be spasmodic (i.e., allergic) or bacterial. As many as 75% of cases of viral croup are caused by parainfluenza viruses; other viruses include respiratory syncytial virus, influenza viruses, and adenovirus. Mycoplasma pneumoniae also can cause croup. (See Chapter 189, Parainfluenza Viruses, for more information on croup.)
Viral croup has an insidious onset after a few days of an upper respiratory tract infection. It progresses to hoarseness and the characteristic inspiratory stridor and barking cough. Symptoms wax and wane and usually are worse at night. Oral intake may be decreased.
Spasmodic croup appears as a sudden onset of severe stridor, usually at night, in a well child with no upper respiratory tract infection. The cause is thought to be allergic, but viruses may play a role.
Bacterial croup presents much like viral croup, but with higher fever and with more severe respiratory distress. If an affected child is intubated, pus is found in the trachea. Staphylococcus aureus is the most common organism; Streptococcus pneumoniae, Streptococcus pyogenes, and Haemophilus influenzae type b (Hib) also are isolated. Bacterial croup may be a superinfection of viral croup.
Clinical Manifestations and Complications
Usually, children with croup are mildly or moderately ill but rarely appear toxic. Such children demonstrate signs of an upper respiratory tract infection and a low-grade fever. Children with croup have a characteristic barking cough and a hoarse voice. The respiratory examination may show signs of upper respiratory obstruction: inspiratory stridor, suprasternal and intercostal retractions, and an increased respiratory rate.
Diagnosis
Croup is diagnosed on clinical grounds. No diagnostic laboratory tests are available. If the diagnosis is in question, a lateral neck radiograph may be helpful to rule out other causes of upper airway obstruction, such as epiglottitis, foreign-body aspiration, or retropharyngeal abscess. Usually, the lateral neck radiograph in croup shows a normal epiglottis, narrowing of the subglottic trachea, and ballooning of the hypopharynx. A posteroanterior neck view shows tapered narrowing of the subglottic air column, known as the steeple sign.
Children with croup should have oxygen saturation checked, because some with severe disease may be hypoxic due to airway obstruction, parenchymal infection, ventilation-perfusion mismatch or, rarely, pulmonary edema.
Therapy
Children with a barking cough and no respiratory distress can be managed as outpatients with extra fluid intake and a cool mist vaporizer or exposure to outdoor air.
All children with croup should be treated with steroids. The administration of one dose of dexamethasone by the oral or intramuscular route (0.6 mg/kg; maximum, 12 mg) decreases inflammation in the upper airway, reduces severity of symptoms within 6 hours, and may decrease the need for other therapies and later hospitalization. Oral and intramuscular administration have equal efficacy, but the oral route is usually preferable because of ease of administration. Dexamethasone suppresses
inflammation for 2 to 3 days after one dose; therefore, further steroid therapy is not necessary. An alternative is to give prednisone initially (2 mg/kg) and to continue the dose daily for 3 days.
inflammation for 2 to 3 days after one dose; therefore, further steroid therapy is not necessary. An alternative is to give prednisone initially (2 mg/kg) and to continue the dose daily for 3 days.
Nebulized budesonide, a synthetic glucocorticoid with strong topical antiinflammatory effects, can be used as an alternative or an addition to oral dexamethasone. It has effects similar to intramuscular or oral glucocorticoids. Nebulized dexamethasone is not as effective as the oral or intramuscular routes, and it is not recommended.
In children with stridor at rest, epinephrine, a local vasoconstrictor given by nebulization, temporarily relieves airway obstruction by decreasing edema in the inflamed subglottic region. Although racemic epinephrine has traditionally been the nebulized form of epinephrine used, L-epinephrine works equally as well, is more readily available, and is less expensive. Because the obstruction often returns to pretreatment level in 1 or 2 hours, affected children must be observed carefully for 3 or 4 hours after this treatment, and then may be discharged in the absence of a relapse of significant respiratory distress.
A mixture of helium and oxygen (heliox) has been used in children with severe respiratory distress from various airway problems, but has not been shown to have any advantage over nebulized epinephrine in children with severe croup. Antibiotics are not indicated except on evidence of bacterial disease. Most patients with croup have excellent short and long-term prognoses; artificial airway intervention rarely is necessary.
Epiglottitis
Epiglottitis, or supraglottitis, is an acute, rapidly progressive, life-threatening airway emergency. Although rare, this condition must be considered in all pediatric patients with an acute febrile illness and symptoms of upper airway obstruction. Cellulitis and edema of the epiglottis, aryepiglottic folds, and hypopharynx narrow the glottic opening. During inhalation, when the structures are pulled inward, stridor and difficulty in breathing occur. During expiration, the airway structures are pushed away, and the glottis is opened. If the edema progresses, the airway may become obstructed completely, and an artificial airway must be established immediately. Pulmonary edema may lead to ventilation-perfusion mismatch and hypoxia.
Pathophysiology
In the past, epiglottitis was an infection caused almost exclusively by Hib, and it affected children most commonly at approximately 3 years of age (range, 2 to 7 years). Since conjugate vaccines for Hib have been developed and widely distributed, the incidence of epiglottitis has decreased by more than 90%. The disease is affecting older children and adults, and the causative organism most commonly is group A beta-hemolytic streptococci. Other organisms include Moraxella catarrhalis and S. pneumoniae. Since 1990, only approximately one-fourth to one-third of cases of epiglottitis have been caused by Hib. Although the age of onset and the organism causing epiglottitis have changed, the clinical presentation, laboratory, and radiographic findings have remained consistent. The incidence peaks during spring and late fall.
Clinical Manifestations and Complications
Typically, children who are older than 2 years of age with epiglottitis present with an acute febrile illness of less than 24 hours’ duration. They may complain of a sore throat and show progressive respiratory distress. On physical examination, often the fever is in excess of 39°C, and the child appears anxious and toxic. If the airway is compromised, the patient sits forward with the neck extended and chin thrust out. Such patients may have difficulty in swallowing and may be drooling. Respiratory signs include a hoarse cough, tachypnea, inspiratory stridor, retractions, and cyanosis. If epiglottitis is suspected, the throat should not be examined, and the child should be left undisturbed. The clinical presentation of epiglottitis in children younger than 2 years of age is more variable and may mimic viral croup. Such young children may have low-grade fever, a history of upper respiratory tract symptoms, and a croupy cough.
Diagnosis
Laboratory and radiographic results may be helpful in the diagnosis of epiglottitis but are not essential early diagnostic tools. A lateral neck radiograph is necessary only if the clinical presentation is not straightforward and the patient is stable. If radiography is indicated, a physician experienced in difficult airway management should accompany the child. Classically, the lateral neck radiograph shows a thumb-shaped epiglottis and narrowing of the posterior airway. Frequently, a complete blood count reveals an increased leukocyte count with a shift to the left. Cultures of the blood and epiglottis, performed in the operating room, often reveal the causative organism.
Occasionally, croup may be confused with epiglottitis and vice versa. Table 116.5 shows the main differentiating characteristics of these two diseases. Croup is diagnosed much more frequently than is epiglottitis.
Therapy
Epiglottitis can be managed with few complications after early suspicion and rapid treatment. Physicians who first see affected children must act quickly to prevent complete airway obstruction. If such children are seen at a tertiary-care center, immediate involvement of ED physicians, an anesthesiologist, an otolaryngologist, and the pediatric intensive care staff is essential. If affected children first are seen by a private pediatrician in an office or clinic, available support staff should be contacted, and arrangements should be made for transport. Children should not be transferred without an accompanying physician prepared to manage the airway.
Affected children should be allowed to assume the most comfortable position, and oxygen is supplied by mask or is blown by the face. The physician or staff must not agitate patients by restraining them, examining the throat, drawing blood, or starting an intravenous line. As soon as possible, children are transported to the operating room, are anesthetized, and are intubated. Usually, the supraglottic structures are inflamed, and culture specimens are taken. Blood can be drawn
for culture and complete blood count, and intravenous antibiotics are given. If the airway is obstructed completely at any time and intubation is not possible, an emergency cricothyrotomy is performed.
for culture and complete blood count, and intravenous antibiotics are given. If the airway is obstructed completely at any time and intubation is not possible, an emergency cricothyrotomy is performed.
TABLE 116.5. DIFFERENTIATION BETWEEN CROUP AND EPIGLOTTITIS | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Studies have indicated that some older children with epiglottitis may be more successful at managing their secretions and may not require intubation. Children with minimal respiratory distress and little or no drooling may be able to be managed with careful observation in a pediatric intensive care unit.
Most cases of sudden death from epiglottitis can be avoided with rapid diagnosis and management. However, some deaths from epiglottitis occur in patients if the disease follows a rapid course and respiratory obstruction occurs before medical care can be obtained or in patients with secondary complications, such as septic shock.
Retropharyngeal Abscess
Retropharyngeal abscess (RTA) is a potentially lethal infection of the deep-neck space, anterior to the prevertebral layer of the deep cervical fascia. Often, the process starts with cellulitis of the posterior pharyngeal wall and progresses to abscess formation. It is most common in children younger than 5 years of age, when the retropharyngeal space contains multiple lymph nodes that drain the nasal cavity, nasopharynx, and sinuses.
Pathophysiology
Although RTA in children usually is a result of the lymphatic spread of infection from the respiratory tract, it also can result from a penetrating foreign body or other trauma, or from contiguous spread from vertebral osteomyelitis. Often, the RTA is polymicrobial, with aerobes and anaerobes. The predominant organisms are S. pyogenes, S. aureus, and oropharyngeal anaerobic bacteria. The differential diagnosis includes epiglottitis, foreign-body aspiration, vertebral osteomyelitis, and lymphoma.
Clinical Manifestations and Complications
Usually, children with RTA have histories of pharyngitis or upper respiratory infection and a sudden onset of high fever. Such patients may have sore throat or difficulty in swallowing; small children may refuse to eat. Breathing may be noisy and, if the abscess impinges on the larynx, affected children will have stridor. Such children may be drooling and have a toxic appearance with the neck extended. Torticollis or meningismus may be present. Usually, cervical lymphadenopathy is evident. On direct examination, a bulge may be seen in the posterior pharyngeal wall, often unilaterally.
The most common complication of RTA is airway obstruction, and affected children must be observed carefully during treatment. Because the retropharyngeal space is contiguous with the mediastinum, direct extension of infection can ensue downward into the mediastinum, causing mediastinitis. The abscess also can rupture, with aspiration that causes respiratory distress or pneumonia.
Diagnosis
If children are seen early in the process of cellulitis and abscess formation, the diagnosis can be difficult. If RTA is suspected, and the child is stable, a lateral neck radiograph is obtained. The optimal film is obtained in the inspiratory phase and in moderate cervical extension. This view shows widening of the prevertebral space at C2; it also may show loss of the normal cervical lordosis and presence of air in the soft tissues. Because obtaining optimal films is difficult in a young child and the retropharyngeal soft space changes appearance rapidly with respiration and crying, interpreting measurements of the space width is difficult. If the diagnosis is questionable or if surgery is planned, a computed tomographic (CT) scan is obtained.
CT with contrast can be used to differentiate between retropharyngeal cellulitis and abscess. It also provides accurate information about the exact location and extent of the abscess and its relation to the great vessels. Color-flow Doppler ultrasonography also may be helpful in evaluating abscess formation.
Therapy
In the event of significant respiratory distress, emergent airway management is essential. Most children with RTA are stable and can be managed with intravenous antibiotics. Antibiotics are chosen to cover normal oropharyngeal flora, penicillinase-producing S. aureus, and Bacteroides species. For a fluctuant abscess, surgical drainage is performed by percutaneous aspiration or by incision.
Peritonsillar Abscess
Peritonsillar abscess (PTA) is an acute accumulation of purulent material between the tonsillar capsule and the superior constrictor muscle of the pharynx.
Epidemiology
The most common deep-space infection of the head and neck, PTA is the most common sequela of acute tonsillitis. Although it can be seen in the first decade of life, it occurs most commonly in adolescents and adults.
Pathophysiology
Antibiotic treatment of tonsillitis does not always prevent the development of PTA, and affected children may be receiving antibiotics when the abscess forms. Usually, cultures of aspirates from the abscess are polymicrobial, including aerobes and anaerobes. The most common organisms are group A beta-hemolytic streptococcus, S. aureus, H. influenzae, and such anaerobes as Bacteroides species, viridans streptococci, and Fusobacterium necrophorum. The differential diagnosis includes epiglottitis, peritonsillar cellulitis, foreign-body aspiration, dental infections, and neoplasms.
Clinical Manifestations and Complications
Usually, children or adolescents with PTA complain of severe unilateral throat pain and have a history of preceding or current pharyngitis. Affected individuals may have difficulty in speaking, swallowing, or opening their mouths, and they speak with a “hot potato” voice.
On physical examination, affected patients may appear toxic, may exhibit a high fever, and may be drooling because of difficulty in swallowing; often, torticollis or trismus is evident. Markedly tender cervical adenopathy is present. The involved tonsil is markedly inflamed and edematous and usually is bulging inferiorly and medially. The uvula may be pushed toward the opposite side. Affected children may be dehydrated from inability to take fluids.
The most common complication of PTA is airway obstruction. In the event of respiratory compromise, the abscess can be drained in the ED with a needle and syringe. Other complications include rupture and aspiration, ulceration of the large submaxillary arteries, sepsis, and mediastinitis.
Diagnosis
If differentiation between peritonsillar cellulitis and abscess is difficult, ultrasonography or contrast CT studies are helpful. Ultrasonography, either externally on the neck or intraorally, can distinguish reliably the early stage of peritonsillitis from well-established abscess formation and can locate the abscess accurately. If PTA is suspected in young, uncooperative children with fever and drooling, or in patients with severe trismus, CT with contrast also is helpful in localizing and defining an abscess.
Therapy
The most important part of the management of PTA is drainage of the abscess. Usually, needle aspiration is the initial drainage procedure. After appropriate sedation and local anesthesia, aspiration is performed by an otolaryngologist. This procedure cures more than 90% of cases of PTA. If the abscess does not resolve, the otolaryngologist may choose to perform an intraoral incision and drainage or acute tonsillectomy. In children with a history of recurrent tonsillitis and an increased risk of recurrence of PTA, the otolaryngologist may choose to perform a tonsillectomy.
The medical management of PTA, along with drainage, includes hydration, pain relief, and antibiotic treatment. Usually, culturing the aspirate is not necessary, and antibiotics are chosen to cover the aforementioned organisms. After abscess drainage, cooperative adolescents often can be treated as outpatients with antibiotics and pain medication. In younger children, usually intravenous hydration and antibiotics are necessary.
Asthma
Asthma, or reactive airway disease, is a chronic condition with acute exacerbations of inflammation, bronchospasm, mucosal edema, and mucus production, all of which contribute to widespread airway narrowing and various degrees of airway obstruction. Children have status asthmaticus if they are unresponsive to initial treatment or have a significant chance of suffering from respiratory failure without vigorous further treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



