12 Screening women
The Principles of Screening
What is screening?
Screening involves the systematic, population-wide recruitment and application of a test to symptom-free individuals considered to be at sufficient risk of a specified disorder to benefit from further investigation or direct preventive action.1 In other words, screening can be defined as the application of diagnostic tests or procedures to asymptomatic people for the purpose of dividing them into two groups: those who have a condition that would benefit from early intervention and those who do not.2
What constitutes an ideal screening program?
Internationally recognised screening principles published under the auspices of the World Health Organization provide guidelines by which to evaluate screening programs. These are listed in Box 12.1.
BOX 12.1 Criteria for the implementation of a screening program
(From Hart106)
The disease
Breast Cancer Screening
Why should we bother screening for breast cancer?
Breast cancer is the most common malignancy in women, comprising 18% of all female cancers worldwide.3 Approximately one million new cases are diagnosed in the world each year, with the average lifetime risk of breast cancer in the USA at birth being 12%, or one in eight.4
The risk of developing breast cancer increases with age, beginning in the fourth decade of life. The probability of developing invasive breast cancer over the following 10 years is 0.4% for women aged 30–39, 1.5% for women aged 40–49, 2.8% for women aged 50–59, and 3.6% for women aged 60–69.5 This high prevalence, together with studies showing that most women with symptomatic breast cancer could not be cured by local surgery,6,7 prompted the search for a mechanism to screen women for early disease.
While breast cancer mortality is clearly higher in the developed world, it is still the most common cancer in women in developing countries. The epidemiology of breast cancer is complex. Major risk factors include age, geographical variation, age at menarche and menopause, age at first pregnancy, family history, previous benign breast disease and radiation, fat intake and hormone use.4
As the evidence from research became apparent in the 1970s and 1980s, many countries established breast cancer screening guidelines and programs. The UK government initiated screening for breast cancer in 1987 by a single, medial–lateral oblique view of each breast every 3 years for all women aged 50–64 years. The breast screening program in Australia—BreastScreen—began in 1991 and provides two-view mammographic screening at 2-year intervals, mainly for women aged 50–69 years. The US PreventiveServices Task Force revised its recommendations in November 20098 and now recommends that:
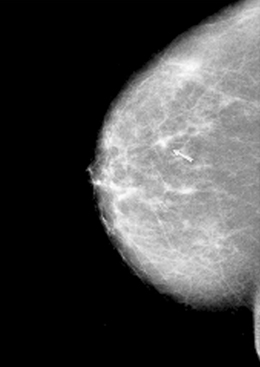
An example of a screening mammogram showing breast cancer is given in Figure 12.1.
What is so controversial about breast cancer screening?
What is the evidence?
Until recently, and after heated controversy, there appeared to be general acceptance that the benefit of screening for breast cancer with mammography had been well documented.9 Large randomised trials, covering a total of half a million women, had been carried out in New York, USA10; Edinburgh, Scotland11; Canada12; and Malmö13, Kopparberg14, Östergötland, Stockholm15 and Göteborg16 in Sweden.
These trials focused primarily on comparing mortality from breast cancer in those women invited to be screened with women who had had no intervention. The most quoted study supporting the efficacy of mammography is a meta-analysis published in The Lancet in 199317 of the Swedish trials. This review found the largest reduction of breast cancer mortality (29%) in women aged 50–69 at randomisation. In women aged 40–49, a non-significant 13% reduction was demonstrated. Screening had only a marginal impact in women aged 70–74 years. Another review published in 1995 in the Journal of the American Medical Association was supportive of these figures, quoting a mortality benefit approaching 30% in women over 50 years of age 7–9 years from the start of the trials.18
In 2000, another meta-analysis of the randomised controlled trials in mammography was published in The Lancet, this time by Gøtzsche & Olsen.19 With the perilous title ‘Is screening for breast cancer with mammography justifiable?’, the authors reviewed the methodology of each of the eight trials in meticulous detail. They concluded that there were significant problems in the randomisation procedures of several of the trials and that only two of the eight trials were therefore valid. When the results of these two trials were then pooled, the outcome showed no significant difference in either total mortality or mortality secondary to breast cancer in those screened with mammography compared with controls. This work was subsequently included in the Cochrane database.20 Nystrom et al updated their review of the Swedish RCTs and answered some of the criticisms levelled at these trials in the Cochrane review. Their results confirmed a 21% (RR = 0.79, 95% CI 0.70–0.89) reduction in breast cancer mortality overall, with the reduction being greatest in the 60–69 year age group (33%).21 The most recent review22 has determined that mammography brings about a reduction in breast cancer mortality of 20%; however, the effect is lower in the highest-quality trials and a more reasonable estimate is a 15% relative risk reduction. Based on the risk level of women in these trials, the absolute risk reduction was 0.05%.
Should a mammographic screening program carry the warning: ‘screening can damage your health’?
Those who argue against mammography point out the fact that as a screening test it results in a significant amount of morbidity. There is an estimated 30% increase in overdiagnosis and overtreatment, or an absolute risk increase of 0.5%.22 Overdiagnosis refers to the detection of abnormalities that will never cause symptoms or death during a patient’s lifetime. Overdiagnosis of cancer occurs when the cancer grows so slowly that the patient dies of other causes before it produces symptoms or when the cancer remains dormant (or regresses). Because doctors don’t know which patients are overdiagnosed, we tend to treat them all. Overdiagnosis therefore results in unnecessary treatment.23 For every 2000 women invited for screening throughout 10 years, only one will have her life prolonged. In addition, 10 healthy women who would not have been diagnosed if there had not been screening will be diagnosed as breast cancer patients and will be treated unnecessarily.24
Table 12.1 shows the morbidity generated by the UK National Health Service (NHS) screening program in 1 year (1997–98).25 These data demonstrate that, while 5% of all women screened are recalled for further assessment, the number of cancers detected are 5.9/1000.
TABLE 12.1 Screening activity generated by the UK NHS Breast Cancer Screening Program (1997–1998)
| Number of women invited | 1,668,476 |
| Acceptance rate (% of invited) | 75.1% |
| Number of women screened (invited) | 1,252,324 |
| Number of women screened (self/GP referrals) | 97,780 |
| Total number of women screened | 1,350,104 |
| Number of women recalled for assessment | 71,255 |
| Per cent of women recalled for assessment | 5.3% |
| Number of benign biopsies | 2212 |
| Number of cancers detected | 7932 |
| Number of cancers detected per 1000 women screened | 5.9 |
| Number of in situ cancers detected | 1718 |
| Number of invasive cancers less than 15 mm | 3381 |
(From NHS Breast Screening Programme Review25)
The high level of morbidity generated by mammography has been very well demonstrated by Elmore et al.26 They performed a 10-year retrospective cohort study of breast cancer screening and diagnostic evaluations among 2400 women who were 40–69 years old. These women received a median of four mammograms and five clinical breast examinations per woman over the 10-year period. Of the women who were screened, 23.8% had at least one false-positive mammogram, 13.4% had at least one false-positive breast examination, and 31.7% had at least one false-positive result for either test.
Studies have also documented the psychological distress caused by abnormal mammograms and being recalled for further tests. Women with high-suspicion mammograms had substantial mammography-related anxiety (47%) and worries about breast cancer (41%). Such worries affected the moods (26%) and daily functioning (17%) of these women, despite diagnostic evaluation that excluded malignancy.27 In general, women have not been informed about the risk of false-positive results and the accompanying anxiety they generate,28 nor have they been warned about the risk of receiving the diagnosis of carcinoma in situ.29
The other issue that should not be discounted when discussing mammography related morbidity is that many women find mammography very painful.30
Women generally exaggerate the benefits and are unaware of the harms of screening and invitations to women to participate in breast cancer screening reinforce this as they are information-poor and biased in favour of participation.31 Box 12.2 lists the points that such invitations and related pamphlets and websites should emphasise to give a more accurate representation of the risks and benefits of mammography.32
BOX 12.2 Suggested contents of evidence-based leaflet on mammography
(From Jorgensen and Gotzsche32)
What other issues need to be considered when evaluating whether mammography is worthwhile?
The other issues to consider, apart from the morbidity generated by the screening test, is the cost and whether or not the funds would be better directed elsewhere, such as to treatment or at least to research into better treatment. Some have suggested that this should be the case.33
Are any other screening tests effective?
Clinical breast examination should be able to detect lesions >1 cm in size. It is likely that some cancers <1 cm in size will be detectable by clinical examination. This means that if mammography is to be more effective than clinical examination, it has to do so through those 22% of cancers <1 cm in size and the 18% in situ cancers. The question is whether these lesions are clinically significant in terms of causing morbidity or mortality.
The only study to address this issue has been the Canadian National Breast Screening Study.35 It enrolled 40,000 women from 1980–1985 into either clinical breast examination and mammography or clinical breast examination alone. After 13 years of follow-up, there was no sign that mortality was lower in the mammography group.35 The combined group had 107 and the examination-only group 105 deaths attributable to breast cancer, giving a cumulative rate ratio of 1.02 (95% CI, 0.78–1.33).
As far as pick-up rates of breast cancer is concerned:
Although longer follow-up may reveal a benefit, currently there is no evidence to suggest that the detection of non-palpable cancers by mammography contributes to reducing mortality from breast cancer.36 However, the study did highlight the morbidity brought about by mammography, with the rate of biopsy of benign lumps being three times higher in the combined examination/mammography group compared with that in the group undergoing clinical breast examination alone.35
In order to determine whether clinical breast examination can be implemented as a screening tool, however, more research needs to be carried out. This research must be a randomised, controlled trial comparing clinical breast examination on its own with no screening at all. Until this research is carried out, the US Preventive Services Task Force concludes that the evidence is insufficient to recommend for or against routine clinical breast examination (CBE) alone to screen for breast cancer.37
If the implementation of clinical breast examination is also controversial, then what about breast self-examination?
Unfortunately, while breast self-examination (BSE) can detect symptomatic breast cancer at an earlier stage, it does not appear to have any influence on mortality. An American Cancer Society study compared 177,602 women who practised BSE during the preceding 13 years with 272,554 women who did not and found similar breast cancer death rates in the two groups.38 The UK Trial of Early Detection of Breast Cancer (TEDBC)39 involved 300,000 women in eight health districts—two with mammographic screening centres, two where BSE was taught by trained nurses and four where neither form of intervention was available. At 16 years, the relative risk of death from breast cancer in women attending the two screening clinics was reduced by 27% (RR, 0.73; 95% CI, 0.63–0.84), but there was no risk reduction in the two BSE centres (RR, 0.99; 95% CI, 0.87–1.12).
Three randomised trials to evaluate the effect of BSE on breast cancer mortality have been undertaken in St Petersburg and Moscow40 and in Shanghai.41 The final results of the Shanghai study,42 which included more than 250,000 women, found a similar incidence and an identical number of breast cancer deaths among BSE subjects and controls. BSE has however greatly increased biopsy rates, with the number of benign lesions detected in the BSE group being twice those of the controls. These findings indicate that women should be aware of their breasts as part of general body awareness and seek medical help when their breasts look or feel abnormal, but the promotion of regular BSE is not justified.22
Conclusions
Since 1990 there has been a decrease in breast cancer mortality in the order of 30%.43–45 The problem is that this decrease may either be due to screening and early diagnosis or it may be secondary to improved therapy, or both. When the original mammography trials were being undertaken, systemic therapy was not as widespread as it is today, when most women will receive some kind of adjuvant therapy.46
Hopefully, research in the areas of aetiology, optimising therapy and achieving better and longer survival will progress, so that breast cancer will no longer be the scourge that it is today.
Summary of key points
Preventing Cervical Cancer
How big a problem is cervical cancer?
Since the advent and widespread use of the Papanicolaou (Pap) smear—a test that detects asymptomatic preinvasive lesions at the earliest stages—the incidence of cervical cancer has dramatically decreased. With the introduction of the National Cervical Screening Program in Australia, new cases of cervical cancer among women of all ages almost halved, from 13.2 new cancers per 100,000 women in 1991 to 6.9 in 2005. Mortality also halved, from 4 deaths per 100,000 women in 1991 to 2 in 2005.13 However, in many parts of the developing world, cervical cancer continues to cause significant morbidity and mortality.
The greatest burden of disease occurs in developing countries, where unfortunately there are no organised cervical screening programs. In terms of absolute number, the Asian region has an incidence of 265,884 cases of cervical cancer per year.47 In contrast, Australia had 734 new cases of cervical cancer and 221 deaths in 2005.48
Human papillomavirus (HPV) has now been causally related to cervical cancer,49 with HPV DNA detected in at least 95% of cervical cancers (of which HPV types 16 and 18 are the most commonly isolated50). The association between persistent HPV DNA detection and cervical cancer is more than 10 times the association between smoking and lung cancer.51
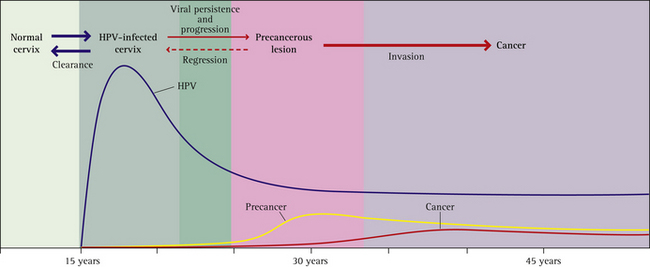
For more information about the natural history of HPV infection see Box 12.3 and Figure 12.2 (p 204).
BOX 12.3 HPV infection: natural history
How can cervical cancer be prevented?
Until recently, the most effective mechanism for cervical cancer prevention was organised cervical cancer screening with a Pap smear. Pap screening detects cellular changes in the cervix caused by persistent HPV infection. If HPV infection is left untreated, it has been estimated that about 30% of women with high-grade lesions would develop cancer over a 30-year period.58 Detection of high-grade lesions through Pap screening allows treatment prior to the development of cancer and as such is a secondary prevention strategy.
HPV types 16 and 18, worldwide, are responsible for about 70% of cervical cancer cases, 50% of high-grade precancerous lesions and 25% of low-grade lesions.59 HPV 6 and 11 infections are associated with most genital warts and around 8–10% of low-grade cervical lesions.60
Who should receive the HPV vaccine?
Administering the vaccines to the pre-adolescent population maximises the chances of most of the population achieving immunity before HPV exposure (i.e. before sexual activity commences). As a more robust immune response to vaccination is achieved at this age, protection is likely to endure through the years of maximal exposure. However, extended follow-up of populations in clinical trials will help to determine whether a booster is required.61
Favourable estimates of the cost-effectiveness of a catch-up immunisation program for women up to the age of 26 years in economic models adapted to Australian data led to federal government funding of a universal immunisation program for girls aged 12 and 13 in Australia, with a 2-year catch-up program for older adolescent and young adult women.57 This catch-up program, run through schools and general practitioners, ended in 2009. Australia was the first country in the world to roll out a national HPV vaccination program and has established a register of women who received the vaccine. This register will facilitate cross-referencing of vaccination data with information from cervical cytology (Pap smear) or cervical cancer registries for evaluation purposes in the future.
Both Cervarix and Gardasil are registered in Australia for women up to the age of 45. Many question the utility of vaccinating women who are already been sexually active and therefore have already been exposed to HPV. However, the following questions remain to be conclusively addressed61:
And until these issues are resolved, vaccination in women up until the age of 45 can be considered to be like taking out ‘insurance’. Advice for ‘older’ women related to HPV vaccination is given in Box 12.4.
BOX 12.4 Advice about HPV vaccination for women up to the age of 45 years
(From Skinner et al61)
Is it too late to vaccinate a woman if she has a history of HPV disease shown by clinical evidence such as an abnormal Pap test or genital warts?
Should women be tested for HPV prior to vaccination?
No. While women who receive the vaccination who are already sexually active are likely to have been exposed to one or more strains of HPV, it is unlikely they have been exposed to both HPV 16 and 18 and therefore the vaccine will still provide them with some protection. Currently there are no validated, approved and readily available HPV type-specific polymerase chain reaction or serological assays.61 Were they available and used, the process would add considerable expense to an already expensive intervention.61
What is the role of HPV vaccination in women who have already had dysplasia?
The HPV vaccine is a prophylactic, not therapeutic, vaccine. It will therefore have no effect on current HPV infection or current or previous dysplasia. Its aim is to prevent the acquisition of new HPV (primarily types 16 and 18, although there is some evidence of cross-protection with other strains).62
Cervical Cancer Screening
Which women should be screened for cervical cancer?
One of the most basic questions to ask when implementing a screening test is who should have it done? To answer this question one needs to have an understanding of the risk factors and pathophysiology of cervical cancer. Traditionally we have been told that all women who are having or have ever had sexual intercourse are at risk of cervical cancer, the reason being that they may have acquired HPV as a result of their sexual activity. This is slightly simplistic, however, especially when considering whether virgins, lesbians and women in monogamous relationships should be screened.
The recent introduction of polymerase chain reaction (PCR) testing has revealed that HPV infections are much more common among young, asymptomatic women than previously suspected. The site-specificity of genital HPV led to the assumption that HPVs were primarily transmitted by sexual contact. However, since HPVs have been detected in virgins, infants and children, and after juvenile laryngeal papillomatosis was shown to be caused by these viruses, there has been acknowledgment of the fact that HPV may be transmitted by other non-sexual routes as well.63 Despite this, women who have never engaged in sexual intercourse with a man have a minimal risk of developing squamous cell cancer of the cervix.
At what age should cervical cancer screening commence?
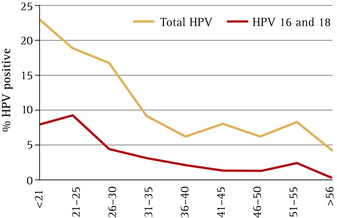
As seen in Figure 12.3, between 20% and 25% of women have acquired HPV in their 20s. There is a steep decline in HPV prevalence up to the age of 35, and after the age of 55 the prevalence is <5%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree