Fig. 40.1
Frequency of primary sites of osteosarcoma according to age. (Data obtained from SEER 17 database) [30]
Clinical Presentation
When all HNOS, pediatric and adult, are considered together, tumors occur most commonly in the mandible (45–49 %) with the maxilla closely following as the second most common site (47–40 %) [17, 23]. In one of the only reports on pediatric HNOS, of 18 individuals the maxilla and mandible were equally involved (44.5 % each) and other sites were involved 11 % of the time [9]. The majority of patients with HNOS present with a mass lesion or swelling which can be accompanied by pain [12, 15]. Trismus is rarely described as an isolated symptom in HNOS, likely because it is almost always accompanied by pain. It is also important to recognize that symptoms of gnathic osteosarcoma may mimic dental infection. In fact, in one study, 44 % of individuals with these tumors presented to their dentist first for presumed tooth etiology [15]. Other rarer signs and symptoms of HNOS can include cranial nerve palsies, proptosis, or increased intracranial pressure [31].
Etiology and Biology
Like with all osteosarcomas, the etiology for most primary osteosarcoma of the head and neck is unknown. The most significant risk factor for HNOS in children is hereditary retinoblastoma. Other risk factors include prior radiation therapy, and additional cancer predisposition syndromes. Li–Fraumeni and Rothmund–Thomson syndromes predispose individuals to osteosarcoma in general but not specifically HNOS. Paget’s Disease of bone also results in predisposition to osteosarcoma; however, because it causes osteosarcoma in older individuals, Paget’s disease will not be discussed here.
Li–Fraumeni syndrome is an autosomal dominant familial condition involving germline mutations of the TP53 gene that manifests with a very high incidence of malignancies, including osteosarcoma. A study of a large database of TP53 mutation carriers published in 2003 demonstrated that 13.4 % of these individuals with tumors had osteosarcoma [32]. However, the literature does not suggest that anatomic distribution of osteosarcoma in Li–Fraumeni syndrome differs from sporadic osteosarcoma.
Rothmund–Thomson syndrome is an autosomal recessive disorder associated with poikiloderma and other skin abnormalities, as well as bone developmental defects. An increased likelihood of osteosarcoma was first shown in 1990. There are no reported cases of Rothmund–Thomson-syndrome-associated HNOS; rather, these are appendicular skeleton tumors [24].
Hereditary retinoblastoma is caused by a heterozygous germline mutation in the RB1 gene, on the long arm of chromosome 13 [1]. In children who suffer from hereditary retinoblastoma, about 50 % of secondary tumors (after occurrence of retinoblastoma) are osteosarcomas [20]. Originally, the increased risk of osteosarcoma in hereditary retinoblastoma was thought to be strictly secondary to DNA damage inflicted by radiation therapy delivered to the orbit to treat retinoblastoma. However, it is now known that the genetic defect in hereditary retinoblastoma contributes to increased osteosarcoma incidence independent of radiation therapy as demonstrated by an increased prevalence of osteosarcoma in patients with hereditary retinoblastoma at sites distant from radiation fields, such as the extremities. Radiation exposure does further increase HNOS risk in hereditary retinoblastoma. Children who have been irradiated for hereditary retinoblastoma therapy are 2,000 times more likely to get osteosarcoma of the skull than the average person, while they are 500 times more likely to develop osteosarcoma of the extremities [26]. Among children and adults with HNOS, a history of hereditary retinoblastoma is common. Four percent of 173 children and adults with HNOS [23] and 33 % of a group of 18 children with HNOS had a history of hereditary osteosarcoma [9]. While retinoblastoma is almost always diagnosed before the age of five, secondary osteosarcoma may not be diagnosed until adulthood .
Secondary osteosarcoma due to radiation from other pediatric tumors of the head and neck, such as leukemia, brain tumors, and other soft tissue tumors, such as rhabdomyosarcoma, does occur in very small numbers, and the latency period is often a decade or more [34]. It is notable that throughout the literature HNOS secondary to radiation is statistically linked to decreased survival compared to other non-radiation-associated primary HNOS, suggesting that this is a more aggressive tumor type [17, 15].
Diagnosis and Staging
Complete assessment of a newly identified head and neck bone tumor with imaging is required prior to biopsy in order to allow for appropriate planning of the best biopsy approach. Plain films are a good initial imaging modality to help identify the bone or region of interest for further evaluation and to define the extent of periosteal new bone formation or osteolysis present, but plain films are of limited utility because superimposed bony structures in the head and neck region permit only crude visualization of mass lesions. On cross-section imaging, osteosarcomas typically appear as a tumor arising from bone, causing cortical destruction and resulting in a soft tissue mass containing calcification. Computerized tomography (CT) shows more details of bony involvement and invasion into surrounding structures, and 3D modeling from CT can be helpful for presurgical mapping of the tumor (Fig. 40.2). Magnetic resonance imaging (MRI) provides the most details of soft tissue involvement [9]. Up to 15–20 % of patients with osteosarcoma have metastatic spread at the time of diagnosis; sites of distant metastases in osteosarcoma are, most commonly, the lungs followed by bones. Chest CT and bone scan are performed as part of staging work up to look for distant metastases [42].
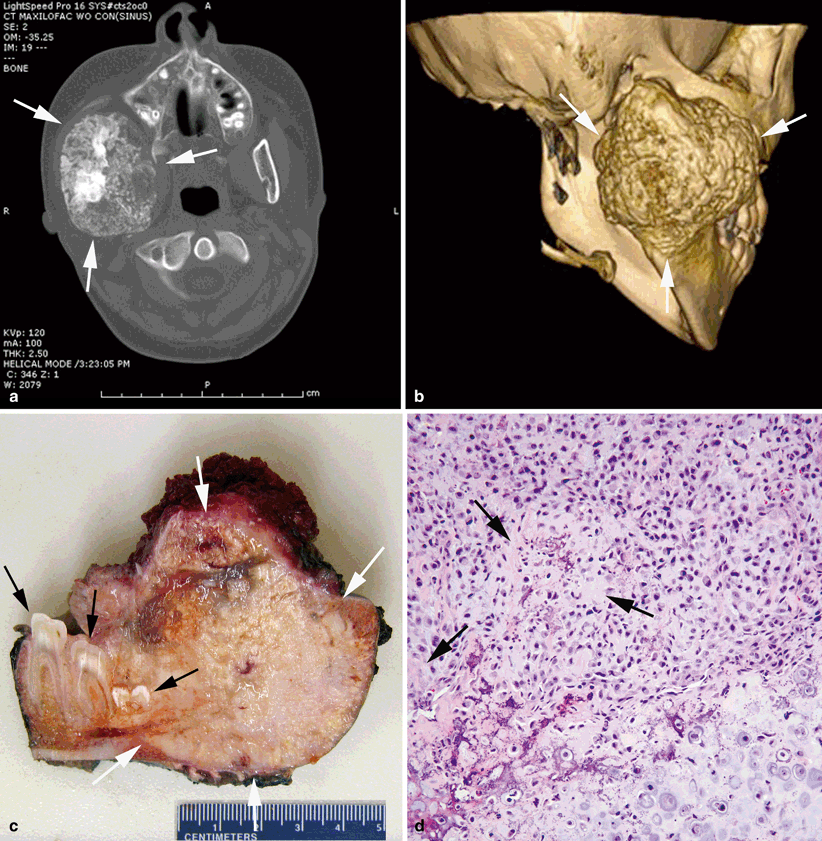
Fig. 40.2
Chondroblastic osteosarcoma of the mandible. a Bone-destructive, irregularly spherical mass centered in the right posterior mandible (between arrows). The mass reveals prominent spotty calcifications. b Three-dimensional reconstruction of the posterior mandibular mass (between arrows). c Cut section of the resected specimen showing the firm, destructive mass (between white arrows) with white-gray color, granular calcifications and areas of necrosis and hemorrhage. Cut section of the molars is indicated by black arrows. d Cellular tumor composed of large atypical cells with focal osteoid formation (arrows). Chondroid matrix is seen on the right lower corner of the photograph
Definitive diagnosis of HNOS requires a tissue biopsy. Surgical open biopsy is the traditional approach for obtaining a tissue biopsy. More recently, interventional radiology-guided, percutaneous, core needle biopsy has become a common approach, especially at tertiary care centers with interventional radiology specialists and large volumes of pediatric solid tumor patients. The diagnostic yield of a core needle biopsy is operator dependent and ranges between 78 and 94 % [4, 22, 41]. More recent studies that benefit from updated technology and user familiarity with the technique demonstrate diagnostic percentages on the higher end of this range. Regardless of the biopsy approach, it is important to sample the soft tissue component of the mass if possible, as this usually provides the greatest diagnostic yield. Surgical biopsy is most often incisional rather than excisional given that neo-adjuvant chemotherapy is often given prior to surgical resection. Osteosarcoma has been reported to recur into the tract left by the biopsy apparatus, so it is essential for the physician performing the biopsy, either percutaneously or surgically, to choose an entry point that will be removed en bloc with the tumor when it is surgically excised [8].
Differential diagnosis of HNOS includes other malignant primary bone tumors, other malignancies involving bone, benign bone tumors, and infectious and inflammatory conditions (Table 40.1). On pathologic examination osteosarcoma is a malignant tumor composed of pleomorphic cells associated with osteoid matrix production. Based on the degree of atypia, differentiation, and necrosis, the tumors can be classified as low, intermediate, or high grade. In children, low-grade tumors are very uncommon. In the head and neck, osteosarcomas are usually rich in chondroid matrix (Fig. 40.2) .
Table 40.1
Differential diagnosis of HNOS
Other malignant primary bone tumors |
Ewing sarcoma |
Chondrosarcoma |
Fibrosarcoma |
Other malignancies presenting as bone tumor(s) |
Lymphoma |
Neuroblastoma |
Metastatic rhabdomyosarcoma |
Metastatic melanoma |
Langerhans cell histiocytosis |
Benign bone tumors |
Aneurysmal bone cyst |
Osteoblastoma |
Osteoid osteoma |
Giant cell tumor |
Unicameral bone cyst |
Hemangioma |
Infectious/inflammatory |
Osteomyelitis |
Chronic recurrent multifocal osteomyelitis |
Natural History
One interesting difference between HNOS and osteosarcoma of the long bones is the difference in propensity for metastases, both at time of diagnosis and following initial surgical and/or medical therapy for the primary tumor. The available case series on these tumors, in both adults and children, suggest that metastasis at time of diagnosis is very rare in primary HNOS, as opposed to in osteosarcoma in general, where 25 % of initial diagnoses are made in the presence of distant metastases. In the St. Jude pediatric cohort, none of the 18 patients with HNOS had distant metastases at time of diagnosis [9]. A small case series of five pediatric patients of St. Louis, published in 1973 concluded that none of their cases had metastasized at time of diagnosis [10]. At M.D. Anderson, too, in a cohort of 12 patients between the ages of 12 and 21 years that were retrospectively examined none had evidence of distant metastases at time of diagnosis [21].
Management
In non-head and neck osteosarcoma the standard treatment approach is a combination of systemic chemotherapy and local control, most often accomplished by complete surgical resection with wide margins. The current and historical data show that with surgery alone, more than 80 % of non-head and neck osteosarcoma will recur with distant metastases because of micro-metastatic disease [26]. Chemotherapy when added to surgical resection has been proven to improve overall survival [25]. However, in HNOS some controversy exists regarding whether to administer chemotherapy due to lack of data concerning the utility of chemotherapy in this disease.
Chemotherapy
Standard of care for chemotherapy treatment of osteosarcoma in sites other than the head and neck is four cycles of treatment with doxorubicin, cisplatin, and high-dose methotrexate and two cycles of treatment with doxorubicin and high-dose methotrexate (Fig. 40.3). This regimen is typically abbreviated as MAP. Some institutions, particularly in Europe, add ifosfamide to MAP and, in doing so, decrease the cumulative dose of doxorubicin given [3, 27]. Typically two cycles of MAP therapy are given prior to surgery in order to facilitate early initiation of chemotherapy and surgical planning, but upfront resection followed by chemotherapy is also an acceptable approach. Because of the differences in the natural history of HNOS compared to all other osteosarcomas, the role of chemotherapy is less certain .
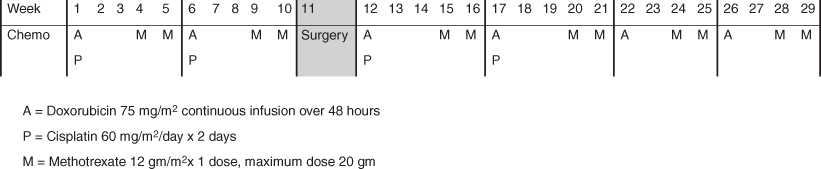
Fig. 40.3
MAP chemotherapy regimen for the treatment of osteosarcoma
As discussed above, HNOS differs in that it metastasizes infrequently. Retrospective studies to evaluate whether the use of chemotherapy impacts survival in HNOS are difficult to interpret due to the inevitable issue of confounding factors. In one study from Memorial Sloan–Kettering Cancer Center that included adults and children who were treated with radical surgery following neo-adjuvant chemotherapy, chemotherapy did not significantly improve event-free survival. However, only patients who were determined to have high-grade tumors, unresectable tumors, or predisposing factors to HNOS such as retinoblastoma were offered chemotherapy in this study [33]. In patients who have positive surgical margins following resection or an unresectable HNOS, retrospective studies suggest that patients receiving chemotherapy have a better outcome; however, the study populations are small [31].
Two meta-analyses of combined adult and pediatric data published in 1997 assessed the role of chemotherapy in HNOS. These studies oppose one another. In the first, only adjuvant chemotherapy was addressed, and the authors concluded that there was no significant difference in 5-year survival between the groups that received chemotherapy (50 %) versus surgery alone; however, the study did not address the question of surgical margins or resectability of the tumors—both important prognostic factors [23]. In the second study, the authors concluded that the addition of chemotherapy led to significantly prolonged survival and better outcomes in general for patients with HNOS, both for individuals who had complete surgical removal and who had incomplete resections. They recommended the same protocol for HNOS as for non-head and neck osteosarcoma [38].
In pediatrics, the practice is generally to offer chemotherapy for HNOS patients, and children with HNOS have been permitted to enroll on Children’s Oncology Group studies of chemotherapeutic regimens in osteosarcoma [14, 27]. In order to determine the impact of chemotherapy in HNOS in children, randomized control trials would be needed but patient numbers are too small to permit studies of this type.
Local Control: Surgery
Because osteosarcoma is relatively resistant to radiotherapy, definitive local control of HNOS, like any osteosarcoma, requires complete surgical resection with negative surgical margins. In non-head and neck osteosarcomas, surgical resectability is an important prognostic factor. For this reason osteosarcoma of the pelvis has a significantly worse outcome than osteosarcoma involving other sites [19]. Similarly, retrospective studies have shown that complete surgical resection of HNOS with negative margins is the most significant prognostic factor influencing overall survival [17, 33, 42]. This includes a retrospective study in a pediatric cohort, where a Kaplan–Meier survival analysis of HNOS patients showed a 75 % 5-year survival of individuals who underwent complete resection as compared to a 35 % 5-year survival of those who underwent incomplete resection or biopsy, regardless of adjuvant therapy [9]. The surgical management of HNOS is complicated by anatomical challenges of resection of the gnathic, neck, and skull bones. Mandibular tumors have the highest rates of negative margins because of ease of surgical access, and therefore have the best outcome, followed by maxillary lesions and skull tumors, which are the most difficult to resect. Therefore, the goal should always be complete removal with negative margins, which, unfortunately, is not always achievable in the head and neck region .
The extent of surgical margin required in osteosarcoma in order to be considered adequate to decrease the risk of local recurrence is a topic of great debate. Marginal and intralesional margins are associated with a poor outcome and an increased risk of local recurrence [5]. In general, orthopedic surgeons treating osteosarcoma of the limb aim for margins of 2–5 mm for soft tissue and 2–3 cm for bone marrow. The pathological/surgical staging system utilized in osteosarcoma is the Enneking staging system (Table 40.2) [11]. Most osteosarcomas in children are high grade (G2) and extra-compartmental, meaning that the tumor has broken through the cortex of the bone. Consequently, most osteosarcomas in children are Enneking stage IIB or III .
Stage | Grade | Site | Metastasis |
|---|---|---|---|
IA | G1 | T1 | M0 |
IB
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|