Fig. 44.1
Histopathology of papillary carcinoma. a Distinct branching papillae with delicate fibrovascular cores and occasional psammoma bodies (arrow). b Nuclei are typically large, crowded (on top of each other), ovoid, clear (“Orphan Annie eye”), and occasionally grooved (arrows). In the inset, nuclear grooves at higher magnification are indicated by arrows
Follicular adenocarcinoma is less common and is difficult pathologically to differentiate from normal thyroid tissue or follicular adenoma.
Medullary carcinoma differs from the other two in that the cell of origin is the parafollicular C-cell (Fig. 44.2). This subtype accounts for 2–5 % of thyroid tumors and arises spontaneously or in association with described cancer syndromes (MEN 2a and 2b, familial medullary thyroid carcinoma (FMTC)) [2, 6].
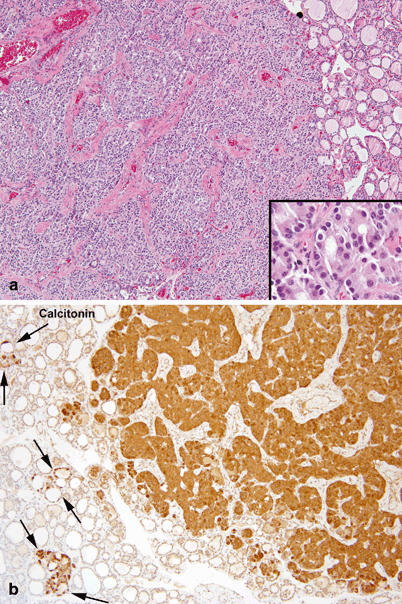
Fig. 44.2
Histopathology of medullary thyroid carcinoma. a Well demarcated nodule of medullary carcinoma composed of large sheets of tumor cells outlined by dense fibrovascular septae. Tumor cells have regular round nuclei with fine chromatin and abundant eosinophilic cytoplasm (inset). b Strong cytoplasmic immunoreactivity for calcitonin in the nodule of medullary carcinoma, in adjacent tumorlets and in foci of C-cell hyperplasia (between arrows)
Rat sarcoma(RAS) proto-oncogene mutations are found in 20 % of papillary cancers and 80 % of follicular cancers [2, 7].
Specific rearranged during transfection(RET) proto-oncogene mutations are associated with the MEN and FMTC syndromes. In addition, mutations in RET are associated with 40 % of sporadic medullary cancer and 35 % of papillary cancers [7, 8].
Additional genetic mutations in the v-Raf (Virus-induced rapidly accelerated fibrosarcoma) murine sarcoma viral oncogene homolog B1(BRAF) oncogene are also associated with papillary cancer, with more recent data suggesting that particular BRAF mutations may be predictive of a more aggressive malignancy.
Other types of primary thyroid neoplasms (e.g., lymphoma) are not discussed here due to their extreme rarity [4].
Incidence and Prevalence
The reported incidence of thyroid nodules in children is approximately 1 % [9].
The incidence of malignancy within thyroid nodules in children is relatively high, ranging from 12 to 36 % in recent reports [9–11].
Overall, thyroid carcinoma accounts for 3 % of pediatric malignancies. Evaluated in the opposite fashion, approximately 10 % of thyroid cancers occur in children [2].
The pediatric population accounts for approximately 2 % of all newly diagnosed thyroid cancers [6].
Age Distribution
Graves’ disease typically occurs in adolescence to early adulthood, though neonatal Graves’ disease in infants of affected mothers is well described.
Hashimoto’s disease incidence peaks during puberty and adolescence.
In children, the peak incidence of thyroid cancer, like that of thyroid nodules, occurs in early adolescence (Fig. 44.3) [2, 11].
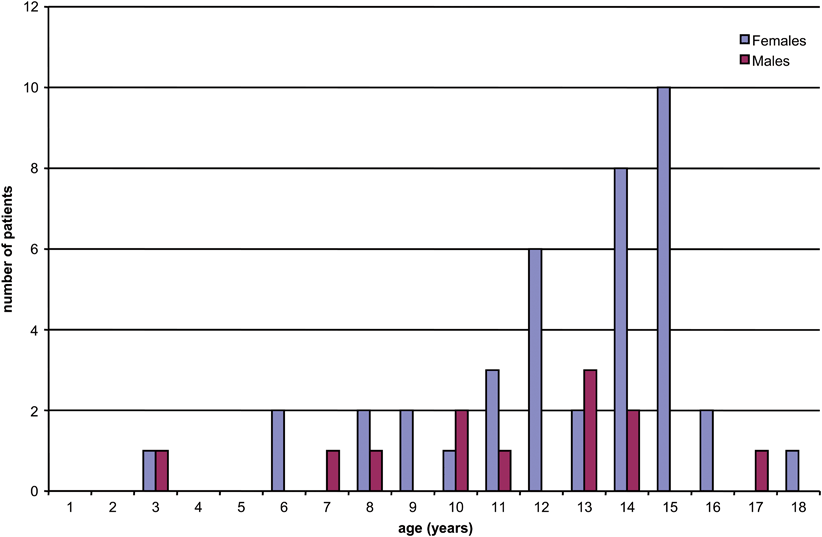
Fig. 44.3
Age distribution of thyroid nodules operated upon at our institution. A similar age distribution exists for pediatric thyroid cancer. (Reprinted from S. Scholz et al. [11], with permission from Elsevier)
Sex Predilection
Geographic/Ethnic Distribution
Higher rates of thyroid cancer are found in adolescent female patients of white and Hispanic backgrounds than black female patients or male patients of any race [6].
Risk Factors
Previous radiation, in the form of directed neck radiation, environmental disasters such as Chernobyl, or previous radiotherapy for other malignancy is related to a higher incidence of thyroid cancer in a dose-dependent manner [6]. The time from radiation exposure to development of thyroid carcinoma is 4–6 years, though the identification of the thyroid malignancy may take longer [2, 12].
Prior malignancy is also related to an increased incidence of thyroid cancer. Thyroid cancer accounts for 9 % of secondary malignancies and is the most common secondary malignancy in survivors of childhood Hodgkin’s and non-Hodgkin’s lymphoma [6]. Conversely, Hodgkin’s disease is the most common primary cancer in patients with secondary thyroid cancer [2, 12].
Relationships to Other Disease States, Syndromes
Medullary carcinoma is the thyroid malignancy associated with FMTC, MEN 2a, and MEN 2b. FMTC is self-explanatory. MEN 2a is comprised of hyperparathyroidism, pheochromocytoma, and medullary carcinoma of the thyroid. MEN 2b is also comprised of pheochromocytoma and medullary thyroid cancer (MTC, without hyperparathyroidism), but also manifests with a marfanoid habitus and cutaneous neuromas.
In the MEN syndromes, medullary carcinoma of the thyroid has nearly 100 % penetrance, is typically the first malignancy to present, and is invariably the most common cause of death. Treatment guidelines are derived from this principle and the genotypic variations that have now been delineated [8].
The PTEN hamartoma tumor syndrome follows a germline mutation in the tumor suppressor PTEN gene. Patients with this syndrome develop benign and malignant growths of multiple tissues, most notably the breast, thyroid, bowel, and skin. The thyroid component can present as either follicular or papillary or both histologic subtypes of thyroid cancer. Recent data suggest that this presentation can occur early, in preteenage to teenage children, rather than adulthood as previously suspected. Patients suspected to have this syndrome should undergo an annual US surveillance from the time of diagnosis [14].
Gardner syndrome, one of the familial adenomatous polyposis (FAP) syndromes, is caused by a defect in the adenomatous polyposis coli (APC) gene. It is characterized by multiple colonic polyps, osteomas and other soft tissue tumors, desmoid tumors, retinal pigmentation, and an increased risk of thyroid cancer. In one series, the average age at which thyroid cancer was diagnosed in patients with Gardner syndrome was 23 years, with many of the patients diagnosed in their adolescent years [4, 15].
Presentation
Symptoms
Most pediatric patients with differentiated thyroid cancer present with an asymptomatic, incidentally noted solitary neck mass due to a thyroid nodule [4].
Recently, as imaging studies become more sensitive, many patients present with thyroid nodules noted incidentally on imaging for trauma or other indications. In addition, nodules are often detected early and incidentally in patients followed for the development of secondary cancers after therapy for a childhood malignancy (e.g., Hodgkin’s lymphoma).
Children present with malignant involvement of the cervical lymph nodes more frequently than adults. Lymph node involvement at diagnosis is present in 40–80 % of children with differentiated thyroid cancer, compared with a 20–50 % incidence in adults. Distant metastases are likewise more common in children, with 7–25 % of children presenting with metastatic disease outside the neck [5–7, 16].
Rarely, patients present with symptoms other than a simple neck mass.
Hoarse voice suggestive of recurrent laryngeal nerve involvement with paralysis of the ipsilateral vocal cord. This is strongly suggestive of malignancy [13].
Swallowing difficulty due to mass effect.
Shortness of breath or dyspnea due to mass effect, especially while recumbent, or due to diffuse pulmonary metastatic involvement. Similar to lymph node involvement, children are more likely than adults to present with pulmonary metastases, in the 20–30 % range [6].
Patterns of Evolution
As mentioned above, the majority of thyroid cancers are detected as solitary nodules. If left untreated, the presentation will evolve to larger primary tumors, potentially with compressive symptoms, cervical lymphadenopathy, and ultimately, possible pulmonary compromise.
Evaluation at Presentation
Evaluation of the child with a neck nodule should include a thorough history geared toward the differential diagnosis, a focused physical examination and appropriate imaging and laboratory studies as indicated below. Emergent presentations are rare.
Differential Diagnosis
Most patients are asymptomatic or have a simple chief complaint of a lump in the neck. Though the differential for this is broad, a more concise differential exists once the lesion is determined to be within the thyroid on US examination. The major diagnoses in this group include :
Benign cyst
Adenoma
Colloid nodule
Hyperplastic nodule
Infectious nodule
Lymphocytic nodule
Differentiated thyroid cancer
Diagnosis and Evaluation
Physical Examination
Physical exam should be focused to evaluate the location of the primary nodule, including mobility with swallowing and freedom from adjacent structures. In addition, assessment for additional nodules, the size of the thyroid gland, and enlarged cervical lymph nodes should be performed. The voice is immediately evident on evaluation of the patient [4].
Findings concerning for malignancy include solitary nodule, evidence of cervical adenopathy in conjunction with a thyroid nodule, fixed lesions, and hoarseness. Compressive symptoms associated with goiter are rarely associated with malignant thyroid nodules .
Laboratory Data
Imaging Evaluation
In children, any neck nodule should prompt a neck and thyroid US, which will localize the nodule. In addition, US characteristics of the nodule can assist in determining the possibility of malignancy. Concerning features include vague margins, calcifications, hypoechoic solid nodules or heterogenous appearance, intranodular vascularity, invasive growth, and suspicious regional lymph nodes [6, 13, 17].
If the TSH is suppressed, an independently active nodule should be suspected and can be confirmed with radionuclide thyroid scintigraphy. Although possible, “hot” active nodules are very rarely malignant and are generally managed with medical therapy targeting the hyperthyroid state [17].
Pathology
Although no consensus pediatric guidelines exist, in general any solid nodule greater than 1 cm in diameter, any nodule with concerning sonographic features regardless of size, and any nodule in a patient with significant risk factors (age, radiation, family history, associated syndrome history) should undergo fine-needle aspiration (FNA) for cytologic evaluation of the nodule. Lesions of borderline size or complex nodules with a significant cystic component may either undergo FNA or be monitored with serial US [17, 18]. If cystic lesions recur after initial aspiration, they should be monitored more closely, sampled with FNA, or be surgically resected for diagnostic purposes [2, 4].
Despite the relatively high risk of malignancy in pediatric thyroid nodules , recent data suggest that the use of FNA, especially US-guided FNA, can beneficially impact the management of pediatric thyroid nodules. Specifically, FNA may preclude the need for surgery in lesions with clearly benign cytology and has been shown to decrease the rate of surgery for benign lesions [6, 9, 17]. Without definite demonstration of efficacy in pediatric patients, however, some authors still recommend immediate surgery for younger patients, whose risk of malignancy is high [2].
FNA is considered cost-effective and highly accurate, in both adult and pediatric patients [4]. This has been demonstrated in multiple single-institution series [6, 9, 11]. A dedicated pediatric meta-analysis found the sensitivity and specificity of FNA for thyroid malignancy to be 94 % and 81 %, with a negative predictive value of over 98 % [19].
The technique for FNA has been evaluated for maximal yield of cellularity without dilution of the sample or excessive blood. In general, US guidance offers the ability to guide biopsy to specific portions of a nodule. This includes the wall, specific solid components of mixed solid and cystic lesions, and areas involved with microcalcifications. Small, generally 25–27 gauge needles are used, with standard syringes to apply suction and collect the specimen. Three back and forth oscillations per second, for 3–5 s per pass, will provide good cellularity without excessive blood. Typically, 2–5 passes per nodule are performed, as tolerated, and each pass should yield 1–2 slides [17]. FNA is generally well tolerated, even by children, but can be made more tolerable with the application of topical anesthetic cream prior to planned biopsy. A variety of slide preparation techniques and processes exist, with prompt slide preparation being the most important factor to ensure adequate assessment and limit unsatisfactory specimens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


