Chapter 190 Salmonella
All Salmonella serovars form a single DNA hybridization group, a single species called S. enterica composed of several subspecies (Table 190-1). Each subspecies contains various serotypes defined by the O and H antigens. To further simplify the nomenclature for physicians and epidemiologists, the names for the common serovars are kept for subspecies I strains, which represent >99.5% of the Salmonella strains isolated from humans and other warm-blooded animals.
Table 190-1 SALMONELLA NOMENCLATURE
| TRADITIONAL USAGE | FORMAL NAME | CDC DESIGNATION |
|---|---|---|
| S. typhi | S. enterica* subsp. enterica ser. Typhi | S. ser. Typhi |
| S. dublin | S. enterica subsp. enterica ser. Dublin | S. ser. Dublin |
| S. typhimurium | S. enterica subsp. enterica ser. Typhimurium | S. ser. Typhimurium |
| S. choleraesuis | S. enterica subsp. enterica ser. Choleraesuis | S. ser. Choleraesuis |
| S. marina | S. enterica subsp. houtenae ser. Marina | S. ser. Marina |
CDC, U.S. Centers for Disease Control and Prevention; subsp, subspecies; ser., serovar.
* Some authorities prefer S. choleraesuis or S. enteritidis rather than S. enterica to describe the species.
190.1 Nontyphoidal Salmonellosis
Pathogenesis
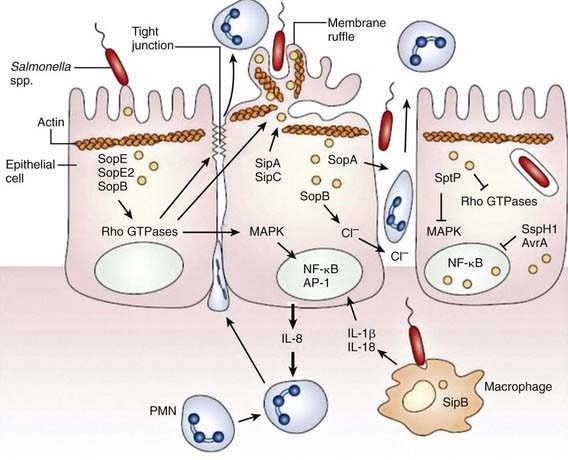
Although S. Typhimurium can cause systemic disease in humans, intestinal infection usually results in a localized enteritis that is associated with a secretory response in the intestinal epithelium. Intestinal infection also induces secretion of interleukin-8 (IL-8) from the basolateral surface and other chemoattractants from the apical surface, directing recruitment and transmigration of neutrophils into the gut lumen and thus preventing the systemic spread of the bacteria (Fig. 190-1).
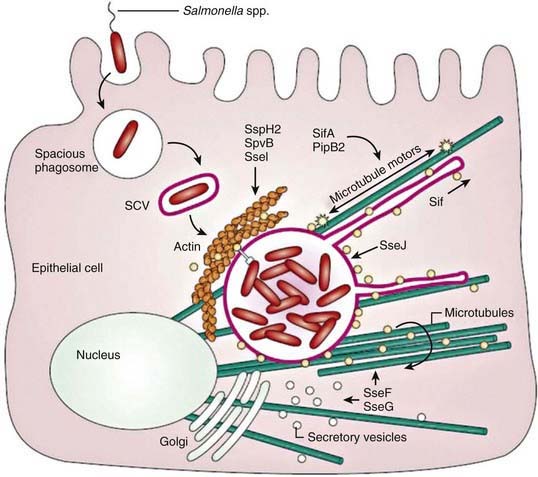
Salmonella species invade epithelial cells in vitro by a process of bacteria-mediated endocytosis involving cytoskeletal rearrangement, disruption of the epithelial cell brush border, and the subsequent formation of membrane ruffles (Fig. 190-2). An adherent and invasive phenotype of S. enterica is activated under conditions similar to those found in the human small intestine (high osmolarity, low oxygen). The invasive phenotype is mediated in part by Salmonella pathogenicity island 1, a 40-kb region that encodes regulator proteins such as HilA, the type 3 secretory system involved in invasion of epithelial cells, and a variety of other products. In humans the TLR-dependent interleukin-12/interferon-λ (IL-12/IFN-λ) is a major immunoregulatory system that bridges innate and adaptive immunity and is responsible for restricting the systemic spread of nontyphoidal Salmonella.
Some virulence traits are shared by all salmonellae, but others are serotype restricted. These virulence traits have been defined in tissue culture and murine models, and it is likely that clinical features of human Salmonella infection will eventually be related to specific DNA sequences. With most diarrhea-associated nontyphoidal salmonelloses, the infection does not extend beyond the lamina propria and the local lymphatics. Specific virulence genes are related to the ability to cause bacteremia. These genes are found significantly more often in strains of S. Typhimurium isolated from the blood than in strains recovered from stool. Although both S. dublin and S. choleraesuis have a greater propensity to rapidly invade the bloodstream with little or no intestinal involvement, the development of disease after infection with Salmonella depends on the number of infecting organisms, their virulence traits, and several host defense factors. Various host factors may also affect the development of specific complications or clinical syndromes (Table 190-2) and of these, HIV infections are assuming greater importance in Africa in all age groups.
Table 190-2 HOST FACTORS AND CONDITIONS PREDISPOSING TO THE DEVELOPMENT OF SYSTEMIC DISEASE WITH NONTYPHOIDAL SALMONELLA STRAINS
Neonates and young infants (≤3 mo of age)
HIV/AIDS
Other immunodeficiencies and chronic granulomatous disease
Immunosuppressive and corticosteroid therapies
Malignancies, especially leukemia and lymphoma
Hemolytic anemia, including sickle cell disease, malaria, and bartonellosis
Collagen vascular disease
Inflammatory bowel disease
Achlorhydria or use of antacid medications
Impaired intestinal motility
Schistosomiasis, malaria
Malnutrition
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree