Primary author (year of publication)
Study duration (years)
Pediatric malignancies (number)
Castro 1972
34
19
Dahlqvist 1982
22
9
Byers 1884
37
26
Baker 1985
25
13
Shikhani 1988
30
3
Lack 1988
58
15
Fonseca 1991
30
7
Callender 1992
43
21
Otago 1994
26
11
Rogers 1994
19
8
Kessler 1994
9
8
Bull 1999
18
5
Orvidas 2000
21
19
Ribeiro Khe 2002
44
27
Yu 2002
25
46
Ethunandan 2003
26
3
De cruz Peres 2004
34
26
Verdine 2006
20
18
Guzzo 2006
30
15
Rahbar 2006
10
7
Ellies 2006
34
9
Castro 1972
34
19
Anatomical Distribution
The most common anatomical site for salivary gland tumors in the pediatric population is the parotid gland [24]. Analysis of the 20 pediatric retrospective studies [13–33] previously mentioned reveals 82 % of the composite 315 tumors to have arisen within the parotid gland; the submandibular gland accounted for approximately 7 %, and the sublingual gland for less than 1 %. The minor salivary glands accounted for the remaining 11 %. These respective incidences are similar to those reported in adults. Rarely the accessory parotid tissue can be the site of origin of a pediatric salivary gland malignancy [34].
Tumor Classification
The histopathology of salivary gland malignancies in the pediatric population is similar to that in adults. The World Health Organization (WHO) has categorized 14 benign and 24 malignant salivary gland neoplasms [4] (Table 39.2). The relative frequency of occurrence of the various histological types does, however, vary. Mucoepidermoid carcinoma is by far the most common in children, accounting for 61 % of the total number of tumors in the twenty retrospective studies [13–33]. Acinic cell (11 %) and adenocarcinoma (10 %) account for a relatively large number, whereas adenoid cystic (9 %), carcinoma ex PA (1 %), and squamous cell carcinomas (0 %) are comparatively rare (Fig. 39.1). Sarcomas, particularly rhabdomyosarcoma, and lymphomas can also arise within the salivary glands in children [35].
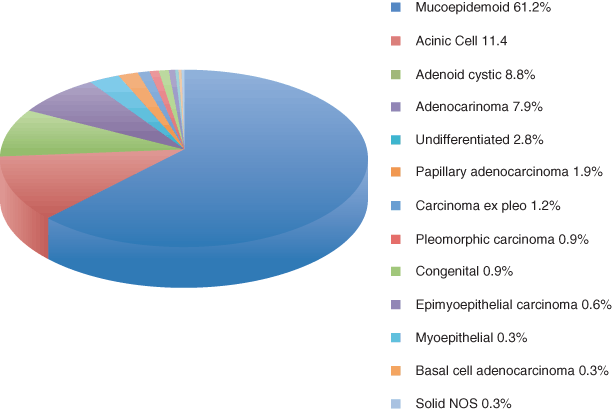
Fig. 39.1
Frequency distribution of malignant salivary gland tumor types in the pediatric population
Malignant epithelial tumors | |
Acinic cell carcinoma (ACC) | 8550/3 |
Mucoepidermoid carcinoma | 8430/3 |
Adenoid cystic carcinoma (AdCC) | 8200/3 |
Polymorphous low-grade adenocarcinoma | 8525/3 |
Epithelial-myoepithelial carcinoma | 8562/3 |
Clear cell carcinoma, not otherwise specified | 8310/3 |
Basal cell adenocarcinoma | 8147/3 |
Sebaceous carcinoma | 8410/3 |
Sebaceous lymphadenocarcinoma | 8410/3 |
Cystadenocarcinoma | 8440/3 |
Low-grade cribriform cystadenocarcinoma | |
Mucinous adenocarcinoma | 8480/3 |
Oncocytic carcinoma | 8290/3 |
Salivary duct carcinoma | 8500/3 |
Adenocarcinoma, not otherwise specified | 8140/3 |
Myoepithelial carcinoma | 8982/3 |
Carcinoma ex pleomorphic adenoma | 8941/3 |
Carcinosarcoma | 8980/3 |
Metastasizing pleomorphic adenoma | 8940/1 |
Squamous cell carcinoma | 8070/3 |
Small cell carcinoma | 8041/3 |
Large cell carcinoma | 8012/3 |
Lymphoepithelial carcinoma | 8082/3 |
Sialoblastoma | 8974/1 |
Benign epithelial tumors | |
Pleomorphic adenoma | 8940/0 |
Myoepithelioma | 8982/0 |
Basal cell adenoma | 8147/0 |
Warthin tumor | 8561/0 |
Oncocytoma | 8290/0 |
Canalicular adenoma | 8149/0 |
Sebaceous adenoma | 8410/0 |
Lymphadenoma | |
Sebaceous | 8410/0 |
Nonsebaceous | 8410/0 |
Ductal papillomas | |
Inverted ductal papilloma | 8503/0 |
Intraductal papilloma | 8503/0 |
Sialadenoma papilliferum | 8406/0 |
Cystadenoma | 8440/0 |
Soft tissue tumors | |
Hemangioma | 9120/0 |
Hematolymphoid tumors | |
Hodgkin lymphoma | |
Diffuse large B-cell lymphoma | 9680/3 |
Extranodal marginal zone B-cell lymphoma | 9699/3 |
Secondary tumors | |
Age and Sex Distribution
Although salivary gland tumors have been reported in children of all ages, the vast majority occur in older children and adolescents. Recognizing the upper age limit varying from 14 to 20 years, composite analysis of retrospective studies identifies a median age of 12 years (28, 31, and 47). One epidemiology study similarly documented an average age of 13.4 years [36]. Benign salivary gland neoplasms present at the slightly older average age of 15 years [17]. Although isolated studies have suggested a strong female predominance (35), composite retrospective article review [13–33] suggests a near equal distribution between the sexes with a 53 % prevalence of salivary gland malignancies in females and a 47 % prevalence in males.
Geographic Distribution
Risk Factors
One of the few well established risk factors for salivary gland cancer is exposure to radiation [10]. The evidence in this respect comes principally from research into the atomic bomb survivors of Hiroshima and Nagasaki, study of whom shows an increased relative risk for benign and malignant salivary gland neoplasms of 3.5 and 11 %, respectively [37]. A strong dose response relationship exists for mucoepidermoid carcinomas [38], and the latent period in salivary tissues was found to be 20 years or more. Data from other studies has also indicated a causative relationship with prior radiation therapy, again with a disproportionately greater representation of mucoepidermoid carcinomas within irradiated patient groups [39].
Second malignant neoplasms in children have emerged as one of the most troublesome complications in pediatric oncology [40]. Over the past few decades, a number of studies have demonstrated a relationship between previously treated childhood cancer, particularly leukemia and lymphoma, and salivary gland cancer [16, 40, 41]. The median latent time between the primary and secondary malignancies was seven years in one study [16], and this was shown by meta-analysis to be significantly shorter in patients treated with both chemotherapy and radiotherapy than radiotherapy alone [42]. It is unclear whether this is a result solely of the cytotoxic treatments or combined to a greater or lesser extent with genetic factors [16].
There is a strong association between Epstein Barr virus (EBV) and lymphoepithelial carcinomas of the salivary glands [43]. No association between salivary gland malignancies and other viruses, such as the human immunodeficiency virus (HIV), polyoma virus and papilloma virus has been convincingly demonstrated [4, 10].
Presentation
The most common presentation of a salivary gland neoplasm is a firm mass in the lateral cervicofacial or submandibular region. A firm salivary gland mass in a child is uncommon, and the fact that over 50 % of these tumors are malignant dictates a thorough diagnostic evaluation by the head and neck surgeon [44]. Unfortunately, the duration from mass documentation until definitive diagnosis varies greatly, ranging in one study from 2 to 156 months [20]. An average time prior to presentation of 8–12 months is often quoted [17, 18, 25]. Parotid gland tumors are usually asymptomatic; rarely adenoid cystic and acinar cell carcinomas may be painful [15, 30]. The incidence of facial nerve paresis on presentation approximates 4 % [16, 19, 21, 22, 26, 30, 33]. Associated regional lymphatic metastases are documented at presentation in 3.5 % children [16, 17, 24, 28]. Intraoral masses in the vicinity of the tonsil or the soft palate can be an uncommon presentation of deep lobe tumors of the parotid. In one review, 1.7 % of patients with parotid tumors were found to present in such a fashion [45].
The usual presentation of submandibular gland tumors is a painless swelling below the mandible. The differential diagnosis includes the much more frequent entities of sialoadenitis of this gland or submandibular region lymphadenopathy. Sublingual gland tumors typically manifest as a palpable fullness in the floor of the mouth. Bimanual palpation of submandibular and sublingual masses may reveal fixation to surrounding structures. Although typically asymptomatic, pain may occur with progressive glandular enlargement. Advanced tumors may involve neural structures including the marginal mandibular branch of the facial nerve, the lingual nerve and the hypoglossal nerve [46] with resultant cranial nerve palsies.
Tumors of the minor salivary glands can be more difficult to diagnose because of their site-dependent presentation. For example, a minor salivary gland malignancy of the hard palate can mimic a bone lesion [47], whereas one of laryngeal origin may produce airway obstruction, dysphagia, or hoarseness. Friability may be an indication of malignancy [17]. The oral cavity accounts for over 50 % of minor salivary gland malignancies in the pediatric population [48]. A painless mass on the palate or floor of mouth is the most common presentation.
Diagnosis and Evaluation
Imaging Evaluation
High-resolution ultrasound is an imaging modality of potential applicability for the evaluation of a parotid or submandibular region mass in a child. Ultrasound can be particularly helpful in distinguishing solid from cystic masses. Doppler ultrasound can evaluate the vascularity of the lesion. Ultrasound has the additional advantages of being noninvasive, not involving radiation, and typically being able to be performed with or without the need for sedation [49]. Any solid mass of the parotid or submandibular region, particularly one without associated inflammatory manifestations, should signal the need for more detailed imaging via computed tomography (CT) or magnetic resonance imaging (MRI) [23]. The age of the child with respect to the need for sedation or general anesthesia may dictate which procedure is initially performed.
Even younger children may tolerate the performance of a relatively short duration CT without sedation. CT is useful in evaluating lymph node metastasis (LNM) and is particularly beneficial if there is a concern of osseous involvement or tumor invasion of neural foramina [50]. Glandular signal intensity and enhancement pattern can, however, parallel that of adjacent musculature on CT. This is particularly true in children, because of the relative lack of fat in the parotid space, soft tissue differentiation in this region may be obscured [41].
MRI, on the other hand, clearly demarcates tumor from glandular tissue in multiplanar fashion. MRI also has the advantage of being free of radiation. T1-weighted images of normal parotid have an image signal intermediate between fat and muscle, whereas submandibular tissue is closer to muscle in intensity [51]. Parotid tumors are well visualized on T1-weighted MR images because of the hyperintense background of the gland. The T1-weighted image gives an excellent assessment of the margin of the tumor, its deep extent, and its pattern of infiltration [52]. Coupled with fat-saturation, contrast enhanced T1-weighted imaging can be used to assess for perineural spread [53]. MR features strongly suggestive of parotid malignancy are poorly defined margins with infiltration into the parapharyngeal space and surrounding musculature [54]. The conventional wisdom is that a hyperintense mass on T2-weighted images is benign and a mass of low to intermediate signal intensity is malignant [52]. A number of studies have, however, shown this not to be a reliable discriminative factor [54]. Innovative techniques, such as diffusion weighted MRI and intravoxel incoherent motion MRI [55] have shown some early promise in distinguishing benign from malignant tumors.
Fine Needle Aspiration or Biopsy
Fine needle aspiration (FNA) cytology in adults is a diagnostic tool that has a reliable sensitivity and specificity for the assessment of salivary gland pathology [56]. Although, its use in children has some advocates [57], the role of FNA is controversial because of the limited established specificity in pediatric salivary gland tumors [58] and because of the need for sedation or even general anesthesia for its performance in younger children [22]. Retrospective study review reveals FNA to be rarely undertaken and the accuracy to be as low as 33 % when it was performed [16].
Incisional biopsy is a consideration in masses involving the tail of the parotid region, and is particularly indicated in clinically unresectable lesions for which a diagnostic biopsy alone is required. Under most circumstances, total excision of the submandibular gland or superficial parotidectomy are the preferred techniques, providing both an excisional biopsy specimen and potential definitive therapy [59]. The marginal mandibular and facial nerves, respectively, need be identified and preserved during these procedures.
Staging
There is no specific staging system for pediatric salivary gland tumors. For parotid, submandibular, and sublingual neoplasms, the adult tumor node metastases (TNM) clinicopathologic staging system unified between the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) is often applied (Table 39.3; [60]). For minor salivary gland malignancies, the staging system for squamous cell carcinomas, depending on tumor site (oral cavity/oropharynx/nasal cavity/nasopharynx) is alternatively used. Children are often diagnosed at an earlier stage than adults [11].
Table 39.3
AJCC staging of salivary gland malignancies
T | Primary tumor | ||
TX: | Primary tumor cannot be evaluated | ||
T0: | No evidence of a tumor | ||
T1: | Tumor 2 cm or less at its greatest dimension, without extraparenchymal extension | ||
T2: | Tumor more than 2 cm but less than 4 cm, without extraparenchymal extension | ||
T3: | Tumor larger than 4 cm, and or tumor with extraparenchymal extension | ||
T4a: | The tumor invades the skin, jawbone, ear canal, and/or facial nerve | ||
T4b: | The tumor invades the skull base and/or the pterygoid plate. And/or encases the carotid arteries | ||
Note | Extraparenchymal extension is clinical or macroscopic evidence of invsion of soft tissue or nerve, except those listed in 4a or 4b. Microscopic evisence alone does not constitute extraparenchymal extension for classification purposes | ||
N | Nodal status | ||
NX: | Regional lymph nodes cannot be evaluated | ||
N0: | No regional lymph node metastasis (LNM) | ||
N1: | Metastasis in a single ipsilateral node, 3 cm or less in greatest dimension | ||
N2: | Metastasis in a single ipsilateral node, more than 3 cm but not more than 6 cm in greatest dimension, or in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension; or in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension | ||
N2a: Metastasis in a single ipsilateral node, more than 3 cm but not more than 6 cm in greatest dimension | |||
N2b: Metastasis in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension | |||
N2c: Metastasis in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension | |||
N3: | Metastasis in lymph node more than 6 cm in greatest dimension | ||
M | Distant metastases | ||
Mx | Distant metastases cannot be assessed | ||
M0 | No distant metastases | ||
M1 | Distant metastases | ||
Stage grouping | |||
I | T1 | N0 | M0 |
II | T2 | N0 | M0 |
III | T3 | N0 | M0 |
T1 T2 T3 | N1 | M0 | |
IVA | T4A, T4B | N0 N1 | M0 |
T1 T2 T3 T4A | N2 | M0 | |
IVB | T4B | Any N | M0 |
Any T | N3 | M0 | |
IVC | Any T | Any N | M1 |
Grade
Owing to the wide range of neoplasms and the diversity of their biological behavior, there is no single grading system for salivary gland cancers. Certain cancers are acknowledged as low grade (for example some adenocarcinomas, basal cell adenocarcinoma, and acinic cell adenocarcinoma) and others as high grade (for example some adenocarcinomas, squamous cell carcinoma, and undifferentiated carcinoma) [60]. Alternatively, salivary gland malignancies have been stratified into low-risk and high-risk tumors, low-risk tumors being those that do not require treatment beyond excision, whereas high-risk being those that do (Table 39.4) [61]. The caveat is that high grade versions of ‘intrinsically’ low grade tumors exist as do low grade versions of typically high grade tumors [61].
Table 39.4
Risk stratification of WHO recognized salivary gland malignancies. (Reprinted from Seethala [61] with permission from Springer Science+Business Media.)
Low risk | High risk |
|---|---|
Acinic cell carcinoma (ACC) | Sebaceous carcinoma/Lymphadenocarcinoma |
Low grade MECa | High grade MECa |
Epitheial-myoepithelial carcinoma | Adenoid cystic carcinomab(AdCC) |
Polymorphous low grade adenocarcinoma | Mucinous adenocarcinoma |
Clear cell carcinoma | Squamous cell carcinoma |
Basal cell adenocarcinoma | Small cell carcinoma |
Low grade salivary duct or cribiform adenocarcinoma | Large cell carcinoma |
Myoepithelial carcinoma | Lymphoepithelial carcinoma |
Oncocytic carcinoma | Metastasizing pleomorphic adenoma (PA) |
Carcinoma ex pleo (low grade/intracapsular) | Carcinoma ex pleo (high grade/widely invasive) |
Sialoblatoma | Carcinosarcoma |
Adenocarcinoma NOS low gradea | Adenocarcinoma NOS low gradea |
Cystadenocarcinoma low grade | Cystadenocarcinoma low grade |
For some of the more common adult salivary gland malignancies, specific grading systems have been utilized. For example, three grades of AdCC have been described based on their growth pattern. The tubular and cribriform patterns are considered low grade, with increasing solid components contributing to higher grade, grade 3 being almost entirely solid [60, 61]. This system is controversial as some authors grade any tumor over 30 % solid as high grade [62], while others deem that up to 50 % of the tumor can be solid and still be termed intermediate [63].
A number of quantitative point scoring grading schemes also exist for mucoepidermoid carcinoma [64]. These assess characteristics, such as intracystic component, presence of neural invasion or necrosis, mitotic rate, and cellular anaplasia to stratify tumors into low, intermediate, or high grade [60]. Most mucoepidemoid carcinomas in children have been found to be low grade, with relative frequencies of low (76 %), intermediate (14 %), and high (10 %) grade (Table 39.5).
Table 39.5
Grading of mucoepidemoid carcinoma. (Reprinted from Auclair et al. [81] with permission of John Wiley & Sons, Inc.)
Author/year | Low grade | Intermediate | High |
|---|---|---|---|
Dahlqvist 1982 | 5 | 0 | 0 |
Byers 1884 | 9 | 4 | 2 |
Baker 1985 | 3 | 0 | 0 |
Lack 1988 | 6 | 0 | 0 |
Fonseca 1991 | 5 | 0 | 0 |
Otago 1994 | 6 | 0 | 0 |
Rogers 1994 | 2 | 3 | 1 |
Kessler 1994 | 8 | 0 | 0 |
Bull 1999 | 1 | 0 | 0 |
Orvidas 2000 | 7 | 1 | 0 |
Ribeiro Khe 2002 | 6 | 6 | 5 |
Ethunandan 2003 | 3 | 0 | 0 |
De cruz Peres 2004 | 18 | 2 | 1 |
Verdine 2006 | 11 | 3 | 2 |
Guzzo 2006 | 9 | 1 | 2 |
Rahbar 2006 | 7 | 0 | 0 |
Ellies 2006 | 3 | 0 | 1 |
Total | 109 | 20 | 14 |
Percentage (%) | 76 | 14 | 10 |
Cellular differentiation has also been used to grade salivary gland malignancies into well, moderately, poorly, undifferentiated, or anaplastic categories. Using this system, a significantly higher percentage of tumors in children (88 %) are reported to be well differentiated or moderately differentiated in comparisom to adults (49 %) [11].
Treatment
Children and adolescents with salivary gland neoplasms should be referred to specialized centers where they can be treated by a multidisciplinary team incorporating pediatric oncologists, radiation oncologists, and head and neck surgeons in order to optimize their management [29].
Surgery
The treatment of choice of most salivary gland neoplasms is complete removal with adequate margins [14]. For submandibular tumors, complete excision of the gland is recommended [59]. In the case of parotid tumors, depending on the lesion, superficial or total parotidectomy is indicated. Parotidectomy is considered a safer and more definitive procedure than tumor enucleation as the latter results in higher rates of recurrence and facial nerve dysfunction. Superficial parotidectomy is the treatment of choice when the tumor is lateral to the facial nerve and subsequent histology reveals a low-grade malignancy. Total parotidectomy is recommended when there is deep lobe involvement, suspected or confirmed high-grade tumors, or tumors with aggressive malignant potential, such as those with facial nerve involvement, multiple intraparotid masses, or cervical metastasis [14, 59]. Definitive surgery allows for very good local control rates approximating 97 % in some series [16, 42].
Resection of the facial nerve is controversial. Factors that historically have influenced facial nerve resection include a tumor completely encapsulating the nerve, large tumors ( > 3 cm), and undifferentiated tumors [18, 31]. Resection is currently recommended only when there is gross anatomic or histopathologic evidence of neural invasion at the time of the surgery [59]. When there is no direct involvement, sparing the facial nerve does not appear to worsen the prognosis [65]. When resection of the nerve is necessary, immediate reanimation by means of primary anastomosis or free nerve graft is advocated [66]. Timely management in this respect is critical for achieving optimal facial function results.
The role of neck dissection in salivary gland cancer in children is also debatable. The overall incidence of LN metastases at presentation for primary parotid carcinomas in all age groups ranges between 18 and 28 % [67, 68]. In the adult literature, recommendations range from routine elective neck dissection in all patients with primary carcinoma of the parotid gland [67] to neck dissection under specific circumstances including patients with tumors larger than 3 cm, high-grade tumors, facial paralysis, extra-glandular extension, and perilymphatic invasion [69]. Others argue in support of a supra-omohyoid neck dissection in patients with high-grade tumors for staging purposes, reserving an elective full neck dissection for patients with undifferentiated and squamous cell carcinomas [70]. In children, simultaneous neck dissection at the time of primary tumor excision is typically recommended only when cervical nodal metastases are clinically detected [15, 20, 23, 26]. Some advocate concurrent neck dissection when there is clinical evidence of high TNM stage or known high histological grade [14]. The clinical picture in children is further complicated by the fact that the presence of lymph node hyperplasia is common, particularly in younger children [59]. Under such circumstances, nodal biopsy is indicated, and a formal modified neck dissection undertaken if there is intraoperative frozen section confirmation of nodal metastases [26, 30, 69].
Permanent facial nerve paresis or paralysis after surgery for benign parotid tumors in adults ranges from 3 to 5 % [71, 72], while transient facial nerve dysfunction ranges from 46 to 65 % [71, 72]. These figures are notably higher when tumor enucleation rather than formal parotidectomy is undertaken [73]. In children, rates of facial nerve palsy following parotidectomy range from 5 to 33 % [11, 15, 18, 21, 30]. This risk of facial weakness may be greater in children compared with adults, with one study reporting 10 of 21 cases had one or more branches of the facial nerve sectioned intraoperatively [26]. This risk is also higher in infants than older children [21]. Preoperative planning for facial nerve grafting should be considered if facial nerve resection is required [14]. As would be expected, complete parotidectomy has higher rates of transient facial nerve dysfunction and secondary Frey syndrome than superficial parotidectomy [74].
Freyʼs Syndrome, also known as gustatory sweating, is characterized by hyperhidrosis and flushing of the lateral cervicofacial skin following parotid surgery. These symptoms are due to abnormal skin innervation by auriculotemporal nerve parasympathetic fibers that communicate with the sympathetic nervous system. Subjective symptoms may be mentioned by 5–50 % of patients, but formal evaluation via the starch iodine test can reveal the presence of this syndrome in the majority of postparotidectomy patients [75]. In children, reports of Frey’s syndrome ranges from 0 to 47 % [15, 18, 21, 22, 76]. Children are typically managed expectantly [76]. In adults botulinum toxin type A injection is the treatment of choice [75].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


