Types of scar rupture: (a) complete rupture; (b) scar dehiscence.
The most important risk factor for uterine rupture is the presence of a previous scar. Other causes are shown in Table 24.1.
| Uterine injury or anomaly sustained before current pregnancy | Uterine injury or abnormality during current pregnancy |
|---|---|
| 1. Surgery involving the myometrium Caesarean section or hysterotomy Previously repaired uterine rupture Myomectomy Deep cornual resection Metroplasty | 1. Before delivery Induction or augmentation of labour External trauma External cephalic version (ECV) |
| 2. Coincidental uterine trauma Abortion Sharp or blunt trauma | 2. During delivery Internal podalic version Difficult forceps Breech extraction |
| 3. Congenital anomaly Pregnancy in undeveloped uterine horn | 3. Acquired in pregnancy Placenta increta or percreta Adenomyosis Sacculation of entrapped retroverted uterus |
Previous Caesarean Section
Antepartum rupture is rare and imaging studies of the previous caesarean scar are unreliable to predict the likelihood of intrapartum uterine rupture [2]. A systematic review that analysed 59 full-text articles including one randomized controlled trial (RCT) reported that the prevalence of rupture ranged from 0.5% to 1% [3].
There is no clear evidence on the effectiveness and safety of the agents used for induction of labour in women with a previous uterine scar. A Cochrane review [4] concluded that there was insufficient evidence available on which to base clinical decisions regarding management. It is, however, widely accepted that the use of misoprostol for induction of labour is contraindicated in the presence of a scarred uterus. One large study (20 095 cases) which analysed women who delivered a second singleton following a previous CS reported a uterine rupture rate of 5.2 per 1000 for spontaneous labour and 24.5 per 1000 for labour induced with prostaglandins [5]. Oxytocin used for induction and augmentation remains an option. However, it has been reported that doses exceeding 20 mU/min increase the risk of uterine rupture at least four-fold [3].
Previous Uterine Surgery
Despite the lack of evidence, the vast majority of obstetricians recommend an elective CS after myomectomy if the cavity has been entered into (i.e. ‘breached’) [6]. The location of the fibroid, the surgical technique and the occurrence of postoperative infection are other factors that can contribute to uterine rupture in subsequent pregnancy [7].
A comparison of the rates of uterine rupture between women with prior myomectomy (176) or prior classical caesarean delivery (455) with women with a prior low transverse caesarean (13 273) showed no statistical difference in the frequency of uterine rupture between the group with a prior myomectomy and the one with low transverse CS [8].
However, this study unfortunately does not state how many patients with a previous myomectomy delivered vaginally.
Laparoscopic myomectomies appear to be safe. A study reviewed 47 pregnancies in 40 patients after laparoscopic myomectomy. Vaginal delivery was attempted in 72% and was achieved in 83% in those who attempted a vaginal delivery with no cases of rupture. The authors advised that vaginal birth can be safely achieved provided they are managed as patients with previous CS [9].
Advances in the subspecialty of fetal medicine have resulted in an increasing number of intrauterine fetal surgeries. Fetoscopic procedures and open procedures such as ex utero intrapartum treatment (EXIT) procedure or mid-gestation open maternal–fetal surgery (OMFS) involve injury to a pregnant uterus and, subsequently, an increased risk of uterine rupture. Wilson et al. [10] reviewed the reproductive outcomes of 97 women undergoing maternal–fetal surgery. The number of subsequent pregnancies was 47, with a uterine dehiscence rate of 14% and rupture rate of 14%. These outcomes in a subsequent pregnancy should form part of counselling prior to OMFS.
Obstructed Labour
This represents an important cause of spontaneous rupture in the developing world, especially in women labouring outside hospital. There is a high incidence of cephalo-pelvic disproportion in Black African women. A retrospective review of 82 cases of uterine rupture in a Nigerian hospital (incidence 0.85%) showed that obstructed labour was the third commonest cause (18.7%) and occurred only in unbooked patients [11].
Multiparity is an independent risk factor for uterine rupture and it is considered to be due to the presence of a greater proportion of collagen compared to smooth muscle.
Congenital Uterine Malformations and Connective Tissue Disorders
In the presence of uterine congenital malformations, the walls are likely to be thinner and tend to diminish in thickness as gestation advances. Moreover, additional thinning can occur in the presence of uterine contractions [12]. Overall, uterine malformations complicate 1 in 594 pregnancies and the greatest risk of uterine rupture occurs during labour.
Disorders of connective tissue can also affect the structure and function of the uterus. There are cases described of uterine rupture associated with Ehlers–Danlos syndrome [13].
Induction of Labour and Termination of Pregnancy
There are only a few RCTs of induction of labour in women with a previous CS. Different methods have different incidence of uterine rupture. Ophir et al. [14] reviewed the existing evidence and concluded that the lowest rate of uterine rupture occurred with oxytocin (1.1%), then dinoprostone (2%), and the highest rate was with misoprostol (6%).
Trauma
Trauma contributes to only a minority of cases of uterine rupture. It usually occurs in the context of a road traffic accident or a history of assault. It is important to optimize education in trauma prevention in pregnancy and exclude uterine rupture in cases of domestic violence [15].
Mechanisms
It is well known that the risk of uterine rupture increases with the use of prostaglandins for induction of labour. However, the exact pathophysiology is not completely clear. Although one of the contributing factors is increased uterine contractility, it is believed that there may also be some biochemical changes within the collagen component of the scar tissue. This is illustrated by the observation that women treated with prostaglandins are more likely to experience rupture at the site of the old scar, whereas women treated with oxytocin experience uterine rupture on sites remote from the old scar [16]. Prostaglandins may induce changes in the collagen and ground substance (glycosaminoglycans) of the uterine scar, predisposing to an increased incidence of scar dehiscence or rupture.
Clinical Features
Uterine rupture can manifest with a wide spectrum of symptoms and signs depending on the site, extent and timing of rupture. While a scar dehiscence can be asymptomatic, a complete rupture can represent a dramatic emergency with fatal consequences for the mother, the fetus or both. Classical symptoms and signs include sudden onset of abdominal pain which is continuous and persistent between contractions, fresh vaginal bleeding, ‘scar tenderness’, evidence of fetal compromise (changes in fetal heart rate (FHR)) and alteration in the shape of the abdomen with the presence of easily palpable fetal parts. It is rare to observe all classical features in a single patient and a high index of clinical suspicion is required.
Abnormal FHR patterns can be detected on a cardiotocograph (CTG). These include cessation of uterine contractions often preceded by tachysystole or hypertonia, reduced baseline variability, variable or late decelerations or a single prolonged deceleration. The mechanisms underlying these CTG features include cord prolapse through the ruptured scar showing variable decelerations and abruption leading to late or prolonged decelerations.
Other symptoms include haematuria and bladder tenderness, especially with a previous lower segment uterine scar, as well as maternal tachycardia and signs of hypovolaemic shock and collapse that can lead to fetal demise or even maternal death, if immediate resuscitation and surgical treatment are delayed.
Uterine rupture presents most commonly as an intrapartum event but it can also occur in the antepartum period and very rarely in the immediate postpartum period.
Antepartum Rupture
Antepartum uterine rupture is characterized by abdominal pain as the most important clinical symptom. Vaginal bleeding may be present, but haemorrhage may be intra-abdominal, resulting in irritation of the diaphragm and causing pain referred to chest or to the shoulder. Antepartum rupture can occur in early pregnancy in patients with previous upper segment scars and not associated with contractions [17].
The patient can present with signs of shock, mainly due to hypovolaemia, although it can also have a neurogenic component. There may be abdominal tenderness, especially if associated with haemoperitoneum or presence of fetal parts into the abdominal cavity; however, uterine scar tenderness is not a reliable sign of uterine rupture.
Intrapartum Rupture
This is the most common presentation of uterine rupture. Abdominal pain is also a common symptom, classically presenting as constant acute pain that doesn’t subside between contractions. Parallel to this, it is possible to observe a loss of contractions on the CTG, usually preceded by tachysystole or hypertonia. It can be difficult to interpret in the context of labour, but should raise the suspicion of uterine rupture or abruption. ‘Scar tenderness’, changes in uterine shape and palpation of fetal parts are other signs suggestive of rupture. They have high sensitivity but low specificity and are frequently unreliable. Vaginal bleeding may or may not occur. Haematuria might be present if there is bladder involvement.
Continuous FHR monitoring is recommended in all women aiming for vaginal delivery after CS (VBAC). Several studies report the association between FHR changes and uterine rupture. Prolonged deceleration, reduced baseline variability and uterine tachysystole were found to be common patterns with uterine rupture [18,19].
A receding presenting part (‘loss of station’) may also be a sign of uterine rupture, if the fetal presenting part had already entered the pelvis prior to the rupture. Abdominal and vaginal examination can identify the presenting part rising above the pelvic inlet.
Postpartum Rupture
This is an extremely rare event that usually presents with abdominal pain and postpartum haemorrhage. On vaginal examination, it is sometimes possible to palpate a dehiscence in the uterine wall and if the rupture is complete the fingers can be passed into the peritoneal cavity. However, studies have shown that systematic manual uterine exploration after VBAC does not improve the outcomes. Moreover, it can increase the risk of manual uterine rupture [20], and therefore this practice should be avoided. Figure 24.2 shows uterine scar dehiscence that occurred during active pushing which was followed by maternal collapse in the immediate postpartum period.
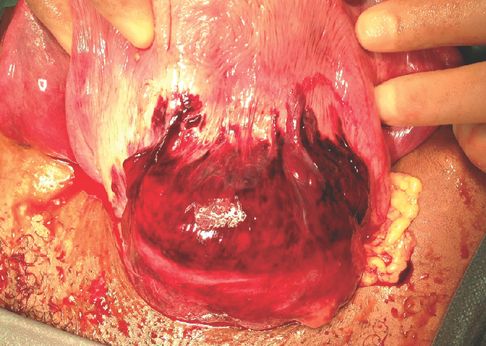
Uterine scar dehiscence during second stage of labour with a haematoma under the visceral peritoneum during laparotomy.
Findings on Laparotomy
Low uterine segment (LUS) is the part most commonly involved in rupture, with some studies reporting up to 92% of cases [21]. However, other parts may be involved, especially on previous classical CS, or involvement of the cervix among patients with an unscarred uterus. Rupture of the lower segment can also extend anteriorly towards the bladder, laterally towards the uterine arteries and into the broad ligament. It is important to perform a systematic examination of the uterus and other abdominal organs to ensure appropriate identification of all areas involved. Posterior rupture is rare but it can occur associated with uterine malformations, obstructed labour or instrumental delivery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


