≥6
27 065
35 619
≥6
15 632
14 488
Position of Vertex
Persistent occipito-posterior position has been associated with increased chance of failure of IOL [8].
Body Mass Index
For reasons still not fully understood, high BMI is a risk factor for failed induction and increases the risk of caesarean delivery [9].
Role of Cervical Length Measurement by Ultrasound and Fetal Fibronectin
Several studies have assessed the predictive accuracy of ultrasound for successful IOL. A study compared transvaginal sonography to Bishop scoring for predicting successful IOL and suggested that cervical length is a better predictor than the cervical score [10]. Cervical length (greater than 20 mm) has been found useful in prediction of the need for CS delivery following IOL. Fetal fibronectin is a basement membrane glycoprotein present in high concentrations in amniotic fluid. It is found in the vaginal fluid before labour and studies have suggested that its presence can predict successful IOL. A study (n = 90) compared cervical clinical data, ultrasound parameters and fetal fibronectin assessment in the prediction of the duration of induced labour when the cervix was unfavourable [11]. Cervical dilatation as assessed by digital examination appeared to be the best predictor of the duration of the latent phase and of that of the whole of labour. Ultrasound measurement of cervical length was not more accurate at predicting the duration of labour than clinical data.
Methods of Induction of Labour
In modern obstetrics, prostaglandins (PGE2 or PGE1), balloon catheters and oxytocin with amniotomy are the most commonly used methods of IOL (Figure 19.1).
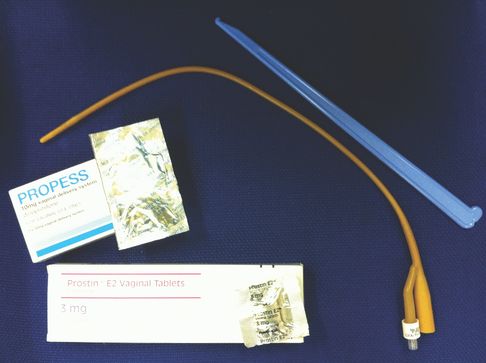
Commonly used methods of cervical ripening/induction of labour (includes slow-release PGE2 pessary, PGE2 pessary, PGE1 tablets, Foley balloon catheter and amniohook).
Pharmacological Methods
Dinoprostone (PGE2)
This is a vaginal prostaglandin that is the most commonly recommended method of IOL with an unfavourable cervix in the absence of any contraindications or risk of hyper-stimulation. PGE2 may be administered as a gel, tablet or slow-release pessary. Each 3 g of gel contains 1 or 2 mg dinoprostone, while tablets contain 3 mg dinoprostone. The first dose is inserted high into the posterior fornix. The patient is then instructed to remain recumbent for at least 30 min. A second and, if required, third dose may be administered at intervals of six hours following cervical assessment. Adverse reactions are uncommon and include vomiting, nausea and diarrhoea. Rarer adverse reactions include uterine hyper-stimulation, fetal distress, maternal hypertension, bronchospasm, backache, rash and, extremely rarely, amniotic fluid embolism. NICE guidelines recommend one cycle of vaginal PGE2 tablets or gel: one dose, followed by a second dose after six hours if labour is not established (up to a maximum of two doses) or one dose of vaginal PGE2 controlled-release pessary over 24 hours [3]. If oxytocin is used after PGE2, six hours should elapse after the last dose of PGE2 to reduce the risk of hyper-stimulation.
Evidence
A Cochrane systematic review in 2014 determined the effects of vaginal prostaglandins E2 and F2α for third trimester cervical ripening or induction of labour in comparison with placebo/no treatment or other vaginal prostaglandins (except misoprostol) [12]. Seventy randomized controlled trials (RCTs) (11 487 women) were included. Overall, vaginal PGE2 compared with placebo or no treatment probably reduced the likelihood of vaginal delivery not being achieved within 24 hours. The risk of uterine hyper-stimulation with fetal heart rate (FHR) changes was increased (4.8% versus 1.0%; risk ratio (RR) 3.16; 95% confidence interval (CI) 1.67 to 5.98; 15 trials, 1359 women). The CS rate was probably reduced by about 10% (13.5% versus 14.8%; RR 0.91; 95% CI 0.81 to 1.02; 36 trials, 6599 women). The overall effect on improving maternal and fetal outcomes (across a variety of measures) was uncertain. PGE2 tablets, gels and pessaries (including sustained-release preparations) appeared to be as effective as each other. The authors concluded that prostaglandin PGE2 probably increased the chance of vaginal delivery in 24 hours. There was an increase in uterine hyper-stimulation with fetal heart changes but this did not affect the CS rates. There was increased likelihood of cervical change, with no increase in operative delivery rates. Another Cochrane review in 2008 that included 56 trials (7738 women) reported that intracervical prostaglandins were effective compared to placebo, but appeared inferior when compared to intravaginal prostaglandins [13].
Recent WHO guidelines also provided further evidence in support of PGE2 and confirmed that PGE2 preparations are more effective than placebo for IOL at term [1]. Low-dose PGE2 has been compared with high-dose protocols and the use of lower doses seems to have comparative advantages like: (a) lower risk of uterine hyper-stimulation with FHR changes; and (b) a trend towards reduced risk of neonatal admission to an intensive care unit. The WHO thus recommends low doses of vaginal prostaglandins for IOL.
Gel versus Tablets
A UK-based RCT involving 165 women compared vaginal PGE2 gel versus tablets for IOL. The mean induction to delivery interval was significantly shorter in women who received the gel (1400 min, 690–2280 min, versus 1780 min, 960–2640 min; p = 0.03). The rate of failed IOL was significantly higher in women who received tablets (10.84 versus 1.22%; p = 0.01). There were no differences in adverse maternal and neonatal outcomes. The authors concluded that PGE2 vaginal gel is superior to vaginal tablets for IOL [14].
Misoprostol (PGE1)
Misoprostol is a PGE1 prostaglandin available for use as tablets, which may be administered through oral, vaginal or rectal routes for IOL. It is available in the form of 200 mcg tablets. While WHO recommends oral misoprostol in a dose of 25 mcg, two-hourly, other authors have advocated use of low-dose vaginal misoprostol, i.e. 25 mcg 3–6 hourly [15]. It is suggested that rather than breaking the 200 mcg tablet into eight pieces using a pill cutter, the tablet should be dissolved into 200 ml of water and 25 ml of that solution be administered as a single dose [1]. In the third trimester, in women with a dead or an anomalous fetus, oral or vaginal misoprostol are recommended for IOL [1]. Misoprostol is, however, not recommended for IOL in women with previous CS. The United States Food and Drug Administration has not yet approved use of misoprostol for induction of labour.
Common side-effects of misoprostol include diarrhoea, vomiting, shivering and pyrexia.
Evidence
Compared with either placebo or expectant management, vaginal misoprostol has been associated with a reduced risk of not achieving vaginal birth within 24 hours of labour induction (RR 0.51; 95% CI 0.37–0.71; five trials, 769 participants) [1]. When compared with intravenous oxytocin alone, vaginal misoprostol has a reduced risk of vaginal births not being achieved within 24 hours (RR 0.62; 95% CI 0.43–0.9; nine trials, 1200 participants), fewer CSs (RR 0.76; 95% CI 0.60–0.96; 25 trials, 3074 participants) and fewer infants with Apgar score below 7 at five minutes of life (RR 0.56; 95% CI 0.34–0.92; 13 trials, 1906 participants) [1]. Compared with other prostaglandins, vaginal misoprostol is associated with a higher chance of vaginal birth achieved within 24 hours (vaginal and intracervical PGE2), fewer CSs (vaginal PGE2) and increased risk of uterine hyper-stimulation with FHR changes, but without increased risk of other adverse perinatal outcomes (vaginal and intracervical PGE2). Compared with higher doses of vaginal misoprostol, lower doses (25 mcg six-hourly) are associated with a reduced risk of uterine hyper-stimulation with FHR changes. When oral and vaginal routes of administration were compared, oral misoprostol was associated with lower risk of poor Apgar score at five minutes of life [1]. A 2010 Cochrane review which included 121 trials also concluded that compared to placebo, misoprostol was associated with reduced failure to achieve vaginal delivery within 24 hours. Uterine hyper-stimulation, without FHR changes, was increased. Compared with vaginal PGE2, intracervical PGE2 and oxytocin, vaginal misoprostol was associated with less epidural analgesia use, fewer failures to achieve vaginal delivery within 24 hours and more uterine hyper-stimulation. Compared with vaginal or intracervical PGE2, oxytocin augmentation was less common with misoprostol and meconium-stained liquor more common [16].
Oral versus Vaginal Misoprostol
A Cochrane review in 2014 assessed the use of oral misoprostol for IOL [17]. Seventy-six trials were included. Oral misoprostol as an induction agent was effective at achieving vaginal birth. It appeared to be more effective than placebo, as effective as vaginal misoprostol and resulted in fewer CSs than vaginal dinoprostone or oxytocin. If using oral misoprostol, the evidence suggests that the dose should be 20–25 mcg in solution. Given that safety is the primary concern, the evidence supports the use of oral regimens over vaginal regimens.
Oxytocin
Commercially used oxytocin is a synthetic form of the posterior pituitary hormone. The WHO recommends that when prostaglandins are not available, intravenous oxytocin alone should be used for induction of labour [1]. However, NICE guidelines do not support the use of intravenous oxytocin alone for IOL. Both guidelines acknowledge that there is a higher chance of vaginal birth within 24 hours with use of prostaglandins as compared to oxytocin alone. In clinical practice, in the case of ruptured membranes, intravenous oxytocin is often recommended as an alternative initiating agent to prostaglandins. Oxytocin is used as an intravenous infusion of a dilute solution (10 mU/ml) and has a time to uterine response of 3–4 min. Steady levels are achieved by 40 min. Generally, the dose is titrated with increasing doses administered every 30 min until regular contractions occur of approximately 45 sec to 1 min duration and are three or four in number every 10 min (Table 19.3).
Commonly used regimen of oxytocin infusion
| Time (min) | Dose of oxytocin (milliunits/ min) | Dilution 30 IU oxytocin in 500 ml normal saline (ml/hr) | Dilution 10 IU oxytocin in 500 ml normal saline (ml/hr) |
|---|---|---|---|
| 0 | 1 | 1 | 3 |
| 30 | 2 | 2 | 6 |
| 60 | 4 | 4 | 12 |
| 90 | 8 | 8 | 24 |
| 120 | 12 | 12 | 36 |
| 150 | 16 | 16 | 48 |
| 180 | 20 | 20 | 60 |
| 210 | 24 | 24 | 72 |
| 240 | 28 | 28 | 84 |
| 270 | 32 | 32 | 96 |
Evidence
A Cochrane review in 2009 included 61 trials (12 819 women) and found that when oxytocin inductions were compared with expectant management, fewer women failed to deliver vaginally within 24 hours (8.4% versus 53.8%; RR 0.16; 95% CI 0.10–0.25) [18]. There was a significant increase in the number of women requiring epidural analgesia (RR 1.10; 95% CI 1.04–1.17). Fewer women were dissatisfied with oxytocin induction in the one trial reporting this outcome (5.9% versus 13.7%; RR 0.43; 95% CI 0.33–0.56). Compared with vaginal prostaglandins, oxytocin increased unsuccessful vaginal delivery within 24 hours in the two trials reporting this outcome (70% versus 21%; RR 3.33; 95% CI 1.61–6.89). There was a small increase in epidurals when oxytocin alone was used (RR 1.09; 95% CI 1.01–1.17). When oxytocin was compared with intracervical prostaglandins, there was an increase in unsuccessful vaginal delivery within 24 hours (50.4% versus 34.6%; RR 1.47; 95% CI 1.10–1.96) and an increase in CS (19.1% versus 13.7%; RR 1.37; 95% CI 1.08–1.74) in the oxytocin group. The reviewers concluded that prostaglandin agents probably increase the chances of achieving vaginal birth within 24 hours as compared to oxytocin.
Non-Pharmacological Methods
Mechanical Methods
Mechanical methods for IOL include insertion of a balloon catheter, extra amniotic saline infusion and hygroscopic dilators. The advantages of mechanical methods include a low risk of FHR abnormalities, low risk of hyper-stimulation and other systemic side-effects, and convenient storage; the disadvantages include discomfort during insertion and the potential to cause antepartum haemorrhage due to a low-lying placenta. It appears that in the absence of prelabour rupture of membranes, mechanical methods for IOL do not result in an increased risk of ascending infection and chorioamnionitis.
Insertion of intracervical 30–50 ml Foley catheter filled with saline is the commonest mechanical mode of IOL. The catheter may be inserted using a ring forceps; the balloon is then inflated and the catheter is retracted firmly against the cervix. The balloon results in pressure to the lower segment of the uterus and the cervix, resulting in local release of prostaglandins. Generally, the catheter is inserted, inflated and left in situ for 12–24 hours.
Several other models of balloon catheters have been introduced based on the same principle.
Evidence
A Cochrane review in 2012 determined the effects of mechanical methods for third trimester cervical ripening or induction of labour in comparison with placebo/no treatment, prostaglandins (vaginal and intracervical PGE2, misoprostol) and oxytocin [19]. The review included 71 RCTs (total of 9722 women). Induction of labour using mechanical methods resulted in similar CS rates as prostaglandins, for a lower risk of hyper-stimulation. Mechanical methods did not increase the overall number of women not delivered within 24 hours; however, the proportion of multiparous women who did not achieve vaginal delivery within 24 hours was higher when compared with vaginal PGE2. Compared with oxytocin, mechanical methods reduced the risk of CS.
The WHO recommends the use of balloon catheter for IOL and cites evidence in its support, including a systematic review which evaluated comparison of the balloon catheter with prostaglandins, oxytocin and placebo [1]. Compared with prostaglandins, the balloon catheter was associated with a lower risk of uterine hyper-stimulation with FHR changes (RR 0.51; 95% CI 0.30–0.86; seven trials, 823 participants), and the risk of CS with the two methods was similar (RR 1.01; 95% CI 0.88–1.17; 19 trials, 2050 participants). Compared with oxytocin, the balloon catheter was associated with a lower risk of CS (RR 0.43; 95% CI 0.22–0.83; two trials, 125 participants). In the comparison of balloon catheter plus oxytocin with misoprostol, the combination approach was associated with a higher chance of vaginal birth achieved within 24 hours [1].
PROBAAT – an open-label RCT published in 2011 – aimed to compare the effectiveness and safety of Foley catheter versus vaginal prostaglandin E2 gel for IOL at term [20]. There were 824 women allocated to IOL with a Foley catheter (n = 412) or vaginal prostaglandin E2 gel (n = 412). Caesarean section rates were much the same between the two groups. It was concluded that in women with an unfavourable cervix at term, induction of labour with a Foley catheter is similar to induction of labour with prostaglandin E2 gel, with fewer maternal and neonatal side-effects.
The opinion of professional organizations differs with regard to the use of mechanical methods of IOL. The balloon catheter is recommended by WHO for induction of labour. The combination of balloon catheter plus oxytocin is recommended as an alternative method of IOL when prostaglandins are not available or are contraindicated [1]. NICE guidelines, however, recommend that mechanical procedures (balloon catheters and laminaria tents) should not be used routinely for the IOL, citing limited evidence for their efficacy and possibly increased risk of neonatal infection [3].
Other Methods of Mechanical Induction
These include hygroscopic dilators like laminaria tents which are placed in the cervix and dilate secondary to water absorption. Several dilators may be inserted into the cervix and they expand over 12–24 hours. The evidence regarding the use of hygroscopic dilators is limited and they do not appear to improve the outcome of IOL. Other methods include castor oil, hot baths, enemas, sexual intercourse, breast stimulation, acupuncture, acupressure and transcutaneous nerve stimulation. All of these lack evidence for safety and efficacy.
Amniotomy (ARM – Artificial Rupture of Membranes)
This involves deliberate rupturing of the membranes using an amniohook. This simple procedure may avoid the need for pharmacological intervention. Amniotomy works best when the cervix is dilated at least 3 cm or more and is favourable. In the past amniotomy was often used to prime the cervix and to induce labour. About 60–80% of women may enter labour within 24 hours of rupture of membranes. Amniotomy alone or in combination with oxytocin should not be used as a method for induction of labour unless the use of PGE2 is contraindicated as per NICE guidelines [3].
Sweeping Membranes
This is recommended for reducing formal IOL. The membranes can be stripped from the internal os and the lower uterine segment by passing a finger and sweeping the membranes around the presenting part leading to release of local prostaglandins (Figure 19.2). Membrane sweeping is associated with a shorter time between treatment and spontaneous labour, a reduction in the incidence of prolonged pregnancy and the need for formal induction. It is estimated that to avoid one formal IOL, membrane sweep must be performed in eight women. The membrane sweep is usually offered at 41 weeks in the antenatal clinic before a planned IOL. Some women may find this procedure uncomfortable and they should be aware of the possibility of having blood-stained vaginal discharge for the next 2–3 days. There is no evidence of increased risk to the mother or fetus from this procedure. A systematic review consisting of 22 studies suggested that routine use of sweeping of membranes from 38 weeks of pregnancy onwards does not seem to produce clinically important benefits. When used as a means for IOL, the reduction in the use of more formal methods of induction needs to be balanced against women’s discomfort and other adverse effects [21].
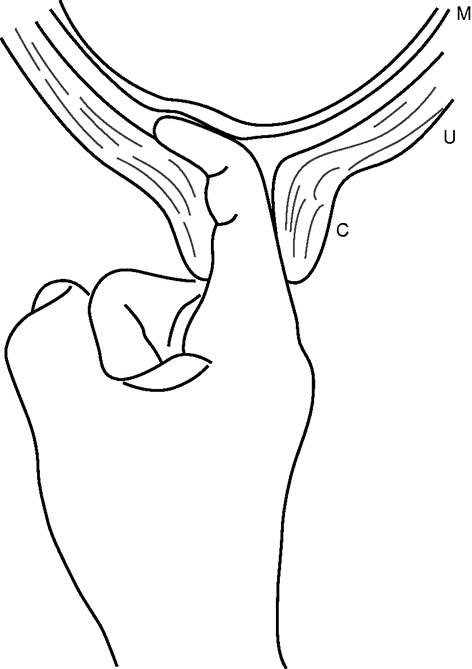
Sweeping of membranes (M) from the cervix (C) and lower uterine segment (U) being performed by the index finger of the examiner.
Mifepristone
Mifepristone is a very effective antiprogesterone and antiglucocorticoid. Randomized trials have shown it to be effective in inducing labour. The use of mifepristone is only recommended following intrauterine fetal death.
The following methods for IOL are not presently recommended for routine clinical practice: oral or intravenous or intracervical PGE2, hyaluronidase, relaxin, corticosteroids, oestrogen and vaginal nitric oxide donors. There is also insufficient evidence to recommend any of the following non-pharmacological methods of IOL: herbal supplements, acupuncture, castor oil, homeopathy, sexual intercourse, curries, enemas and hot baths. It is suggested that nipple stimulation may reduce the number of women not in labour within 72 hours compared to no treatment, but is less effective than oxytocin for this outcome. More research is needed to evaluate the safety of nipple stimulation [22].
Indications for Induction of Labour
Some of the common indications for IOL are listed in Table 19.4. There are also some ‘soft’ indications for IOL, including: previous rapid delivery, poor obstetric history, psychosocial factors or relative geographic isolation. Most recent clinical studies argue against IOL for these indications, citing lack of good-quality evidence of benefit for the fetus or the mother. Caution is advised when considering IOL in cases of multiple pregnancies, unstable lie, polyhydramnios, grand multiparous women and previous low transverse CS. The last two are associated with increased risk of uterine rupture (especially with use of prostaglandins). These pregnancies require close fetal and maternal monitoring throughout the induction process. In some women with a transverse or oblique lie, there may be an indication for IOL. In such cases, external cephalic version should be performed and the head guided over the pelvic brim. An intravenous infusion of oxytocin should be started and, when contractions are established, amniotomy should be performed. Such ‘stabilizing induction’ may reduce chances of cord prolapse. Intrauterine fetal death or major fetal anomalies can necessitate IOL. Mifepristone and misoprostol are the agents of choice for IOL in these situations.
| Indications for induction of labour |
| |
| Contraindications for induction of labour | ||
| Maternal contraindications |
| |
| Fetal contraindications |
| |
Risks Associated with Induction of Labour
It is critical to ensure that women are counselled adequately regarding the risks and benefits of the process prior to IOL and that this is documented appropriately in the notes. Risks associated with IOL are listed in Table 19.5.
Risks associated with induction of labour
| Maternal discomfort and increased need for analgesia | |
| Failure of induction and need for caesarean delivery (about 20%) | |
| Water retention and hyponatraemia with prolonged use of oxytocin | |
| Uterine rupture | |
| Placental abruption | |
| Postpartum haemorrhage | |
| Iatrogenic prematurity | |
| Amniotic fluid embolism | |
| Fetal compromise due to uterine hyper-stimulation | |
| Cord prolapse | |
| Chorioamnionitis | |
| Neonatal jaundice with prolonged use of oxytocin |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


