Transvaginal ultrasound probe with disposable needle guide
17.4.3 Vacuum
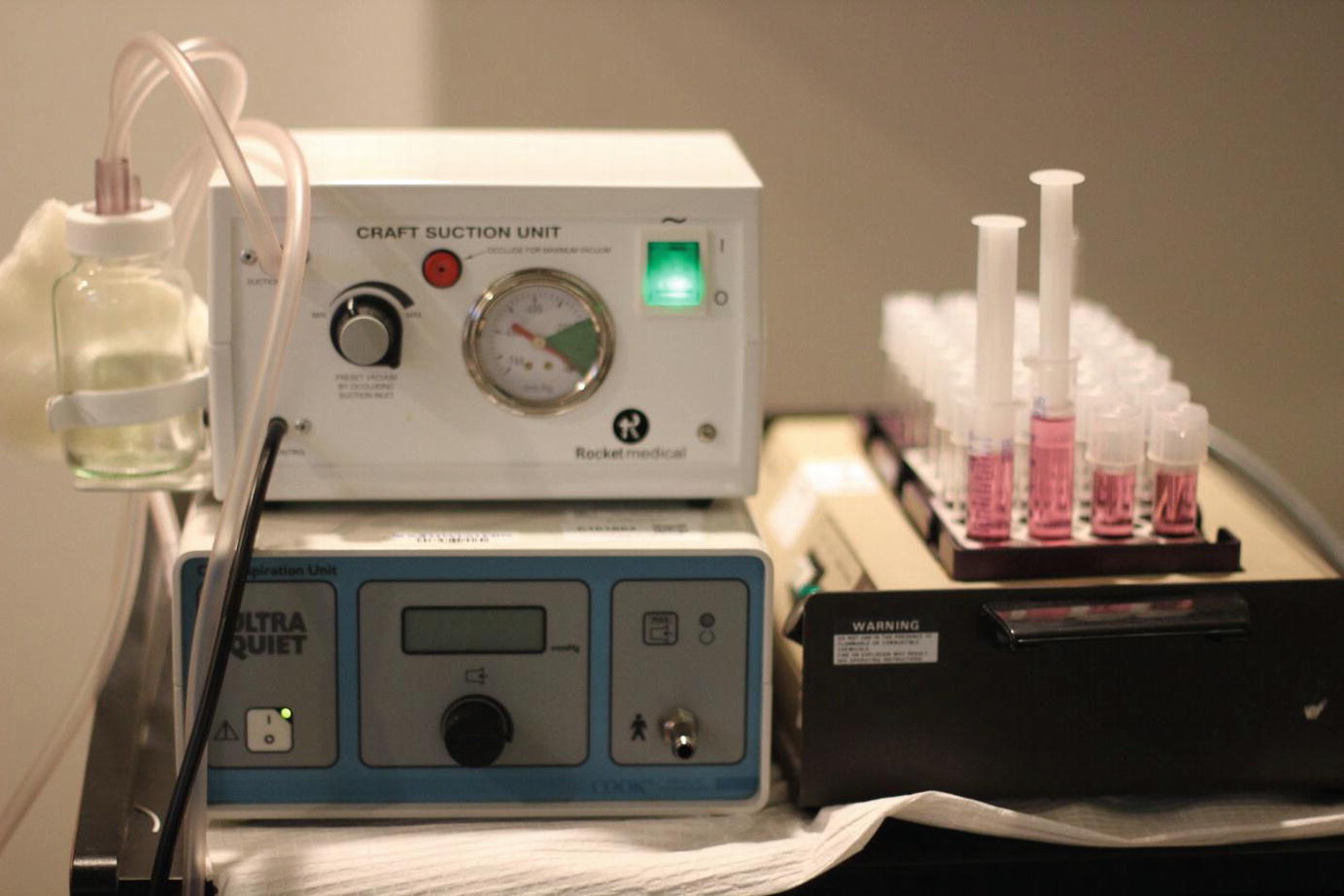
Mechanical aspiration system
17.5 Technique
17.5.1 Patient Positioning
The patient is placed in dorsal lithotomy position in Allen stirrups prior to induction of anesthesia to minimize the exposure of the oocytes to anesthetic agents. The patient should be situated at the end of the operating table, to allow for maximal maneuverability of the ultrasound probe. However, care must be taken to ensure that the sacrum is fully supported by the operating bed.
17.5.2 Bladder Decompression
Patients should empty their bladder prior to oocyte retrieval to decrease risk of needle puncture of the bladder. If the bladder is noted to be full during the initial ultrasound, the bladder should be drained with a catheter. No antibiotics are necessary if catheterization is performed.
17.5.3 Fixation of the Ovary
Using the ultrasound probe, the ovary must be fixed against the pelvic sidewall to ensure successful puncture. If the ovary is not adequately fixed, the needle may further displace the ovary rather than puncture the ovary. Care must be made to ensure that no vessels, bowel, and/or bladder is in between the vaginal wall and the ovary. Color Doppler may be employed to identify vascularity. If the ovary cannot be adequately fixed against the pelvic sidewall, an assistant may provide abdominal pressure to help fix the ovary.
17.5.4 Needle Entry
Faster needle entry should be employed to increase position accuracy and decrease tissue damage. Robotic studies show that the puncture force required and tissue displacement both decrease as the insertion velocity of the needle increases in biological tissues [18]. Thus, it is desirable to enter the follicles with as much velocity as safely possible. For difficult entries in which the ovary is displaced with needle force or the wall of the follicle stretches, drilling into the follicle with gentle rotation may be employed. However, this likely increases the size of the hole in the follicle wall which is less desirable due to potential loss of the oocyte between the follicle wall and the needle. The depth of entry should be approximately two-thirds of the total follicular depth to ensure that the needle is in the follicle for a safe and effective aspiration.
17.5.5 Follicle Curetting
Some practitioners do not move the needle once it is entered into the follicle, while others employ a technique called follicle curetting. This technique involves rotating the needle inside the follicle after complete aspiration of the follicular fluid. Dahl et al. found that follicular curetting significantly increased oocyte yield [19]. An additional benefit may be the result of preventing needle obstruction by a collapsed wall or debris.
17.5.6 Follicle Size
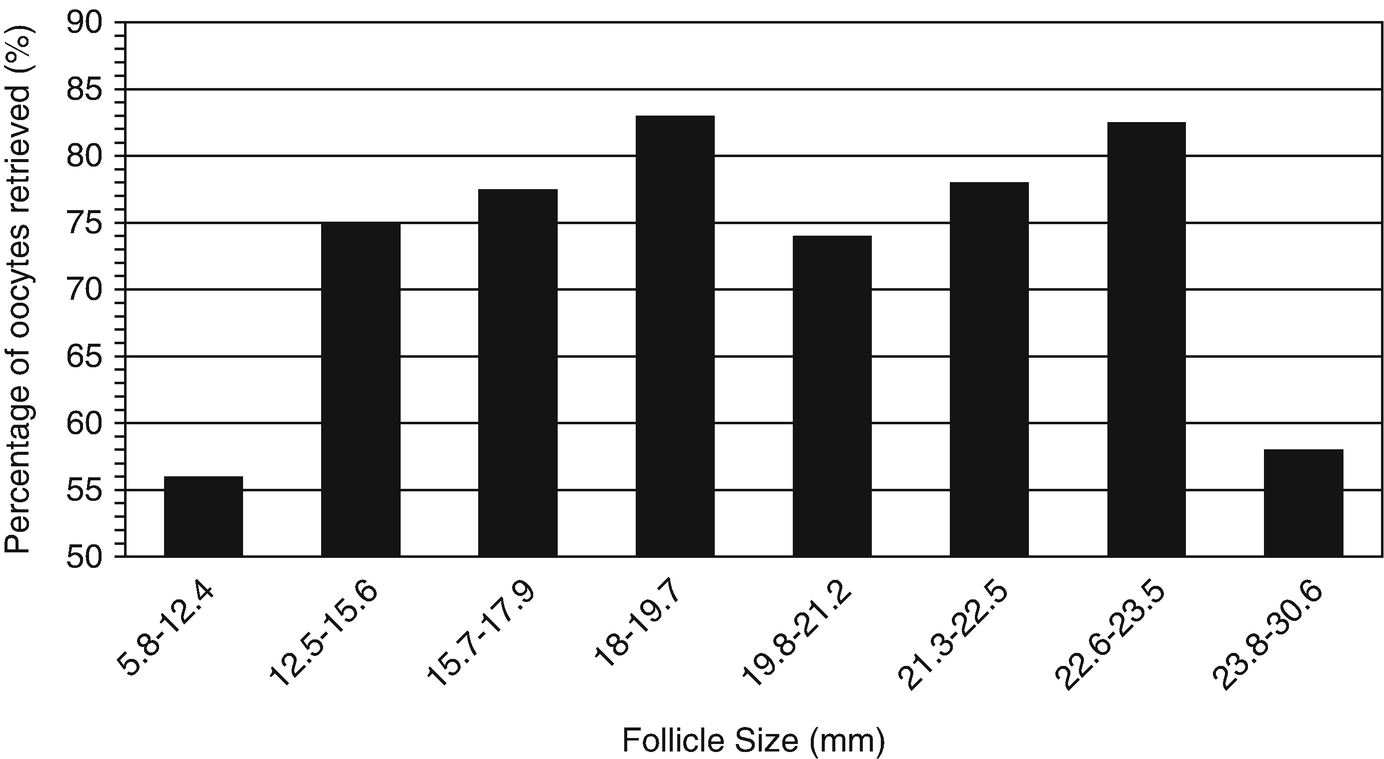
Percentage of follicles from which an oocyte was obtained as function of follicle size. (Modified from Wittmaack et al. [20])
17.5.7 Flushing of Follicles in Minimal and Mild Stimulation Cycles
Follicular flushing has been shown to increase the likelihood of recovering a retained oocyte. Bagtharia and Haloob demonstrated that 40% of oocytes are obtained with a single aspiration, whereas 82% of oocytes could be recovered with two flushes, and up to 97% of oocytes could be retrieved with up to four flushes [21]. In minimal stimulation cycles, it is imperative to obtain every single oocyte due to the low number of expected oocytes per cycle.
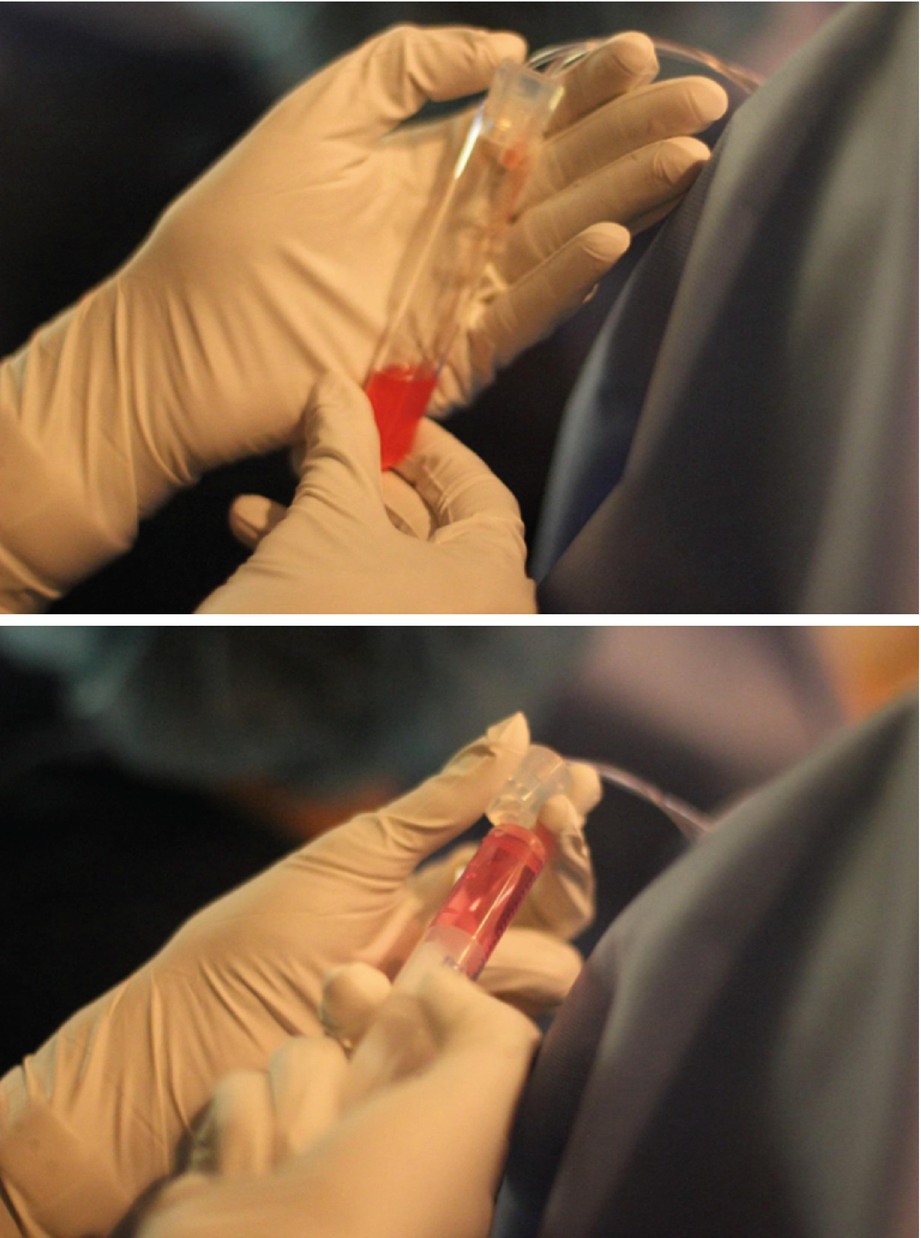
Oocyte collection and flushing
Amount of flush per follicle size
Average diameter (mm) | Media flush (mL) |
|---|---|
10 | 0.5 |
12 | 1.0 |
14 | 1.5 |
16 | 2.0 |
18 | 3.0 |
20 | 4.0 |
22 | 5.5 |
24 | 7.0 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


