Chapter 95 Respiratory Tract Disorders
Respiratory disorders are the most frequent cause of admission for neonatal intensive care in both term and preterm infants. Signs and symptoms of respiratory distress include cyanosis, grunting, nasal flaring, retractions, tachypnea, decreased breath sounds with or without rales and/or rhonchi, and pallor. A wide variety of pathologic lesions may be responsible for respiratory disturbances, including pulmonary, airway, cardiovascular, central nervous, and other disorders (Fig. 95-1).
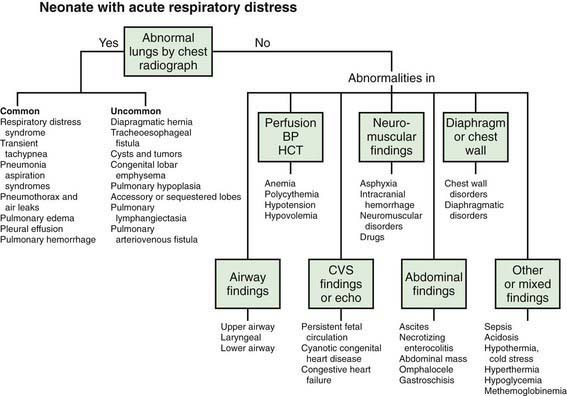
Figure 95-1 Neonate with acute respiratory distress. BP, blood pressure; CVS, cardiovascular system; HCT, hematocrit.
(From Battista MA, Carlo WA: Differential diagnosis of acute respiratory distress in the neonate. In Frantz ID, editor: Tufts University of School of Medicine and Floating Hospital for Children reports on neonatal respiratory diseases, vol 2, issue 3, Newtown, PA, 1992, Associates in Medical Marketing Co.)
95.1 Transition to Pulmonary Respiration
Successful establishment of adequate lung function at birth depends on airway patency, functional lung development, and maturity of respiratory control. Fetal lung fluid must be removed and replaced with gas. This process begins before birth as active sodium transport across the pulmonary epithelium drives liquid from the lung lumen into the interstitium with subsequent absorption into the vasculature. Increased levels of circulating catecholamines, vasopressin, prolactin, and glucocorticoids enhance lung fluid adsorption and trigger the change in lung epithelia from a chloride-secretory to a sodium-reabsorptive mode. Functional residual capacity (FRC) must be established and maintained in order to develop a ventilation-perfusion relationship that will provide optimal exchange of oxygen and carbon dioxide between alveoli and blood (Chapter 415).
95.2 Apnea
Apnea is a common problem in preterm infants that may be due to prematurity or an associated illness. In term infants, apnea is always worrisome and demands immediate diagnostic evaluation. Periodic breathing must be distinguished from prolonged apneic pauses, because the latter may be associated with serious illnesses. Apnea is a feature of many primary diseases that affect neonates (Table 95-1). These disorders produce apnea by direct depression of the central nervous system’s control of respiration (hypoglycemia, meningitis, drugs, hemorrhage, seizures), disturbances in oxygen delivery (shock, sepsis, anemia), or ventilation defects (obstruction of the airway, pneumonia, muscle weakness).
Table 95-1 POTENTIAL CAUSES OF NEONATAL APNEA AND BRADYCARDIA
| Central nervous system | Intraventricular hemorrhage, drugs, seizures, hypoxic injury, herniation, neuromuscular disorders, Leigh syndrome, brainstem infarction or anomalies (e.g., olivopontocerebellar atrophy), after general anesthesia |
| Respiratory | Pneumonia, obstructive airway lesions, upper airway collapse, atelectasis, extreme prematurity, laryngeal reflex, phrenic nerve paralysis, pneumothorax, hypoxia |
| Infectious | Sepsis, meningitis (bacterial, fungal, viral), respiratory syncytial virus, pertussis |
| Gastrointestinal | Oral feeding, bowel movement, necrotizing enterocolitis, intestinal perforation |
| Metabolic | ↓ Glucose, ↓ calcium, ↓/↑ sodium, ↑ ammonia, ↑ organic acids, ↑ ambient temperature, hypothermia |
| Cardiovascular | Hypotension, hypertension, heart failure, anemia, hypovolemia, vagal tone |
| Other | Immaturity of respiratory center, sleep state |
Arad-Cohen N, Cohen A, Tirosh E. The relationship between gastroesophageal reflux and apnea in infants. J Pediatr. 2000;137:321-326.
Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
Kimball AL, Carlton DP. Gastroesophageal reflux medications in the treatment of apnea in premature infants. J Pediatr. 2001;138:355-360.
Litmanovitz I, Carlo WA. Expectant management of pneumothorax in ventilated neonates. Pediatrics. 2008;122:e975-e979.
Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
Ramanathan R, Corwin MJ, Hunt CE, et al. Cardiorespiratory events recorded on home monitors: comparison of healthy infants with those at increased risk for SIDS. JAMA. 2001;285:2199-2207.
Schmidt B, Roberts RS, Davis P, et al. Long term effects of caffeine for apnea of prematurity. N Engl J Med. 357, 2007. 1983–1902
Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112-2120.
Sreenan C, Lemke RP, Hudson-Mason A, et al. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics. 2001;107:1081-1083.
Steer P, Flenady V, Shearman A, et al. High dose caffeine citrate for extubation of preterm infants: a randomized controlled trial. Arch Dis Child Fetal Neonatal Ed. 2004;89:F499-F503.
Tauman R, Sivan Y. Duration of home monitoring for infants discharged with apnea of prematurity. Biol Neonate. 2000;78:168-173.
95.3 Respiratory Distress Syndrome (Hyaline Membrane Disease)
Etiology and Pathophysiology
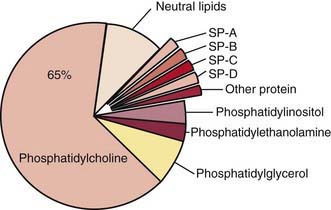
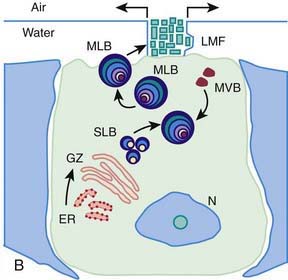
Surfactant deficiency (decreased production and secretion) is the primary cause of RDS. The failure to attain an adequate FRC and the tendency of affected lungs to become atelectatic correlate with high surface tension and the absence of pulmonary surfactant. The major constituents of surfactant are dipalmitoyl phosphatidylcholine (lecithin), phosphatidylglycerol, apoproteins (surfactant proteins SP-A, SP-B, SP-C, and SP-D), and cholesterol (Fig. 95-2). With advancing gestational age, increasing amounts of phospholipids are synthesized and stored in type II alveolar cells (Fig. 95-3). These surface-active agents are released into the alveoli, where they reduce surface tension and help maintain alveolar stability by preventing the collapse of small air spaces at end-expiration. Because of immaturity, the amounts produced or released may be insufficient to meet postnatal demands. Surfactant is present in high concentrations in fetal lung homogenates by 20 wk of gestation, but it does not reach the surface of the lungs until later. It appears in amniotic fluid between 28 and 32 wk. Mature levels of pulmonary surfactant are present usually after 35 wk. Though rare, genetic disorders may contribute to respiratory distress. Abnormalities in surfactant protein B and C genes as well as a gene responsible for transporting surfactant across membranes (ABC transporter 3 [ABCA3]) are associated with severe and often lethal familial respiratory disease. Other familial causes of neonatal respiratory distress (not RDS) include alveolar capillary dysplasia, acinar dysplasia, pulmonary lymphangiectasia, and mucopolysaccharidosis.
(Courtesy of Mary Williams, MD, University of California, San Francisco.)
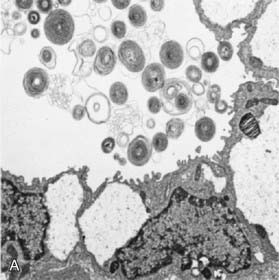
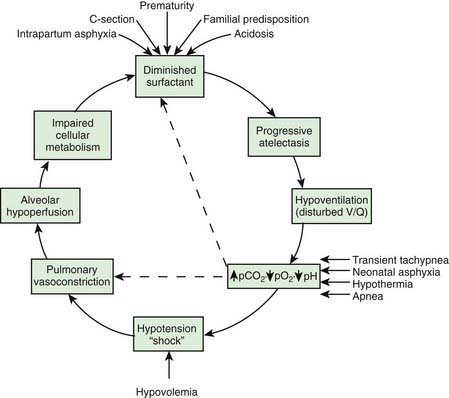
Deficient synthesis or release of surfactant, together with small respiratory units and a compliant chest wall, produces atelectasis and results in perfused but not ventilated alveoli, causing hypoxia. Decreased lung compliance, small tidal volumes, increased physiologic dead space, and insufficient alveolar ventilation eventually result in hypercapnia. The combination of hypercapnia, hypoxia, and acidosis produces pulmonary arterial vasoconstriction with increased right-to-left shunting through the foramen ovale and ductus arteriosus and within the lung itself. Pulmonary blood flow is reduced and ischemic injury both to the cells producing surfactant and to the vascular bed results in an effusion of proteinaceous material into the alveolar spaces (Fig. 95-4).
Diagnosis
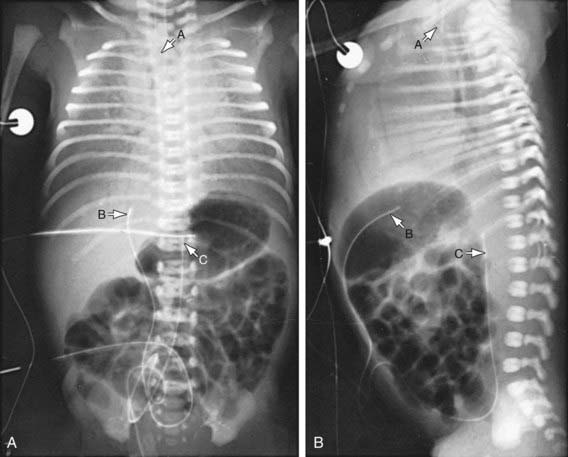
The clinical course, chest radiographic findings, and blood gas and acid-base values help establish the clinical diagnosis. On radiographs, the lungs may have a characteristic but not pathognomonic appearance that includes a fine reticular granularity of the parenchyma and air bronchograms, which are often more prominent early in the left lower lobe because of superimposition of the cardiac shadow (Fig. 95-5). The initial radiographic appearance is occasionally normal, with the typical pattern developing at 6-12 hr. Considerable variation in film findings may be seen, depending on the phase of respiration (inspiratory vs expiratory radiograph) and the use of CPAP or positive end-expiratory pressure (PEEP); this variation often results in poor correlation between radiographic findings and the clinical course. Laboratory findings are characterized initially by hypoxemia and later by progressive hypoxemia, hypercapnia, and variable metabolic acidosis.
In the differential diagnosis, early-onset sepsis may be indistinguishable from RDS. In pneumonia manifested at birth, the chest roentgenogram may be identical to that for RDS. Maternal group B streptococcal colonization, identification of organisms on gram staining of gastric or tracheal aspirates or a buffy coat smear, and/or the presence of marked neutropenia may suggest the diagnosis of early-onset sepsis. Cyanotic heart disease (total anomalous pulmonary venous return) can also mimic RDS both clinically and radiographically. Echocardiography with color-flow imaging should be performed in infants who show no response to surfactant replacement, to rule out cyanotic congenital heart disease as well as ascertain patency of the ductus arteriosus and assess pulmonary vascular resistance (PVR). Persistent pulmonary hypertension, aspiration (meconium, amniotic fluid) syndromes, spontaneous pneumothorax, pleural effusions, and congenital anomalies such as cystic adenomatoid malformation, pulmonary lymphangiectasia, diaphragmatic hernia, and lobar emphysema must be considered in patients with an atypical clinical course but can generally be differentiated from RDS through radiographic evaluation. Transient tachypnea may be distinguished by its short and mild clinical course and is characterized by low or no need for oxygen supplementation. Congenital alveolar proteinosis (congenital surfactant protein B deficiency) is a rare familial disease that manifests as severe and lethal RDS in predominantly term and near-term infants (Chapter 399). In atypical cases of RDS, a lung profile (lecithin:sphingomyelin ratio and phosphatidylglycerol determination) performed on a tracheal aspirate can be helpful in establishing a diagnosis of surfactant deficiency.
Prevention
Avoidance of unnecessary or poorly timed cesarean section, appropriate management of high-risk pregnancy and labor, and prediction of pulmonary immaturity with possible in utero acceleration of maturation (Chapter 90) are important preventive strategies. In timing of cesarean section or induction of labor, estimation of fetal head circumference by ultrasonography and determination of the lecithin concentration in amniotic fluid by the lecithin:sphingomyelin ratio (particularly useful with phosphatidylglycerol measurement in diabetic pregnancies) decrease the likelihood of delivering a premature infant. Antenatal and intrapartum fetal monitoring may similarly decrease the risk of fetal asphyxia; asphyxia is associated with an increased incidence and severity of RDS.
Treatment
The basic defect requiring treatment in RDS is inadequate pulmonary exchange of oxygen and carbon dioxide; metabolic acidosis and circulatory insufficiency are secondary manifestations. Early supportive care of premature infants, especially in the treatment of acidosis, hypoxia, hypotension (Chapter 92), and hypothermia, may lessen the severity of RDS. Therapy requires careful and frequent monitoring of heart and respiratory rates, oxygen saturation, PaO2, PaCO2, pH, serum bicarbonate, electrolytes, glucose, and hematocrit, blood pressure, and temperature. Arterial catheterization is frequently necessary. Because most cases of RDS are self-limited, the goal of treatment is to minimize abnormal physiologic variations and superimposed iatrogenic problems. Treatment of infants with RDS is best carried out in the NICU.
The general principles for supportive care of any premature infant should be adhered to, including developmental care and scheduled “touch times.” To avoid hypothermia and minimize oxygen consumption, the infant should be placed in an incubator or radiant warmer, and core temperature maintained between 36.5 and 37°C (Chapters 91 and 92). Use of an incubator is preferable in very LBW (VLBW) infants owing to the high insensible water losses associated with radiant heat. Calories and fluids should initially be provided intravenously. For the 1st 24 hr, 10% glucose and water should be infused through a peripheral vein at a rate of 65-75 mL/kg/24 hr. Electrolytes should be added on day 2 in the most mature infants and on days 3 to 7 in the more immature ones. Fluid volume is increased gradually over the 1st week. Excessive fluids (> 140 mL/kg/day) contribute to the development of patent ductus arteriosus (PDA) and BPD.
Infants with respiratory failure or persistent apnea require assisted mechanical ventilation. Reasonable measures of respiratory failure are: (1) arterial blood pH <7.20, (2) arterial blood PCO2 of 60 mmHg or higher, and (3) oxygen saturation <85% at oxygen concentrations of 40-70% and CPAP of 5-10 cm H2O. Infants with persistent apnea also need mechanical ventilation. Intermittent positive pressure ventilation delivered by time-cycled, pressure-limited, continuous flow ventilators is a common method of conventional ventilation for newborns. Other methods of conventional ventilation are synchronized intermittent mandatory ventilation (the set rate and pressure synchronized with the patient’s own breaths), pressure support (the patient triggers each breath and a set pressure is delivered), and volume ventilation (a mode in which a specific tidal volume is set and the delivered pressure varies), and combinations thereof. Assisted ventilation for infants with RDS should always include PEEP (Chapter 65.1). High ventilatory rates (60/min) result in fewer air leaks. With use of high ventilatory rates, sufficient expiratory time should be allowed to avoid the administration of inadvertent PEEP.
Surfactant deficiency is the primary pathophysiology of RDS. Immediate effects of surfactant replacement therapy include improved alveolar-arterial oxygen gradients, reduced ventilatory support, increased pulmonary compliance, and improved chest radiograph appearance . Treatment is initiated as soon as possible in the hours after birth. Repeated dosing is given via the endotracheal tube every 6-12 hr for a total of 2 to 4 doses, depending on the preparation. Exogenous surfactant should be given by a physician who is qualified in neonatal resuscitation and respiratory management and who is able to care for the infant beyond the 1st hr of stabilization. Additional on-site staff support required includes nurses and respiratory therapists experienced in the ventilatory management of premature infants. Appropriate monitoring equipment (radiology, blood gas laboratory, pulse oximetry) must also be available. Complications of surfactant therapy include transient hypoxia, hypercapnia, bradycardia and hypotension, blockage of the endotracheal tube, and pulmonary hemorrhage (Chapter 95.13).
Pharmacologic Therapies
Metabolic acidosis in RDS may be a result of perinatal asphyxia and hypotension and is often encountered when an infant has required resuscitation (Chapter 94). Sodium bicarbonate, 1-2 mEq/kg, may be administered over 15-20 min through a peripheral or umbilical vein, followed by an acid-base determination within 30 min, or it may be administered over several hours. Often, sodium bicarbonate is administered on an emergency basis through an umbilical venous catheter. Alkali therapy may result in skin slough from infiltration, increased serum osmolarity, hypernatremia, hypocalcemia, hypokalemia, and liver injury when concentrated solutions are administered rapidly through an umbilical vein catheter wedged in the liver.
Monitoring of aortic blood pressure through an umbilical or peripheral arterial catheter or by oscillometric technique is useful in managing the shock-like state that may occur during the 1st hr or so in premature infants who have been asphyxiated or have severe RDS (see Fig. 94-2). The position of a radiopaque umbilical catheter should be checked roentgenographically after insertion (see Fig. 95-5). The tip of an umbilical artery catheter should lie just above the bifurcation of the aorta (L3-L5) or above the celiac axis (T6-T10). Preferred sites for peripheral catheters are the radial or posterior tibial arteries. The placement and supervision should be carried out by skilled and experienced personnel. Catheters should be removed as soon as patients no longer have any indication for their continued use—usually when an infant is stable and the FIO2 is < 40%. Hypotension and low flow in the superior vena cava (SVC) have been associated with higher rates of CNS morbidity and mortality and should be treated with cautious administration of volume (crystalloid) and early use of vasopressors. Dopamine is more effective in raising blood pressure than dobutamine. Hypotension may be refractory to pressors, but responsive to glucocorticoids, especially in neonates < 1,000 g. This hypotension may be due to transient adrenal insufficiency in the ill premature infant. It should be treated with intravenous hydrocortisone (Solu-Cortef) at 1-2 mg/kg/dose q6-12 hr (Chapter 92).
Because of the difficulty of distinguishing group B streptococcal or other bacterial infections from RDS, empirical antibiotic therapy is indicated until the results of blood cultures are available. Penicillin or ampicillin with an aminoglycoside is suggested, although the choice of antibiotics should be based on the recent pattern of bacterial sensitivity in the hospital where the infant is being treated (Chapter 103).