Respiratory Physiology and Pathophysiology During Sleep
Introduction
As pointed out by previous authors, several factors frustrate attempts to summarize the normal effects of sleep on breathing during postnatal development.1 Although numerous articles explore respiratory system mechanics and respiratory control maturation in sleeping children, there are significantly fewer that explore the effects of sleep, per se, on breathing. Another frustrating reality is the heavily skewed focus on infants, especially preterm infants, while large gaps exist in the literature on sleep and breathing in older infants, children and adolescents. Studies that span the entire developmental age range, from infancy through late adolescence, are rare. The methods used to assess state in these studies vary from full polysomnography to simple observation, often relying on indirect indicators of sleep state. Finally, there is enormous variation in the methods and techniques used to assess respiratory system function, which have evolved over time and vary between studies. In this chapter we will selectively highlight important effects of sleep on normal respiratory physiology during childhood.
Thoracic Cage and Pulmonary Mechanics
Chest Wall Mechanics
Rib cage geometry in infants and children differs markedly from that of adults. Openshaw and colleagues, using chest radiographs and CT scans from individuals 1 month to 31 years of age, found that the dome of the diaphragm and head of the sternum were higher in children, relative to thoracic vertebrae.2 The ribs of infants and young children were more horizontal (less downward slope) compared to older children and adults, and downward slope of the ribs increased with age. These changes occurred primarily between infancy and 2–3 years. The cross-sectional shape of the thorax also changed, being more rounded in infancy and becoming more ovoid (adult pattern) by about 3 years of age.2
In infancy, chest wall compliance is several-fold higher than lung compliance and is even higher, relative to lung compliance, in preterm infants.3–6 With age, chest wall compliance decreases relative to lung compliance; thus, the chest wall becomes stiffer with age while lung compliance changes little. Chest wall compliance becomes approximately equal to lung compliance, as in adults, by the second year of life due to bone ossification and increased muscle mass.4,7
The high chest wall compliance of the neonate has clinical relevance. Passive (relaxed) resting lung volume (Vr) is determined by the balance between the outward recoil of the chest wall and the inward recoil of the lungs. Figure 23-1 shows the static volume pressure curves of the lung (L) and chest wall (CW) typical of a newborn and an adult.8 Note that lung compliance is quite similar at both ages, while the chest wall is much less stiff (more compliant) in the newborn (Fig. 23-1, left panel). When the chest wall is highly compliant, the inward recoil of the lungs (L) is less opposed, resulting in a lower resting lung volume (Fig. 23-1, left panel). As the lungs are the major reservoir for oxygen, low resting lung volume predisposes infants to rapidly developing hypoxemia and atelectasis.9,10
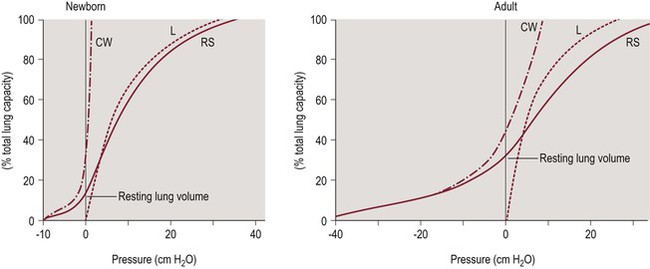
Figure 23-1 Pressure volume curves of the respiratory system.
The solid line represents the compliance of the respiratory system (RS). The chest wall (CW, dash-dot line) is highly compliant in the newborn compared to the adult, while lung compliance (dashed line) changes little with age. Adapted from Agostoni E, Mead J. Statics of the respiratory system. In: Fenn WO, Rahn H, eds. Handbook of Physiology. Washington, D.C.: American Physiological Society; 1964:401.
Paradoxical Inward Rib Cage Motion (PIRCM)
In normal infants, without lung disease or upper airway obstruction, the highly compliant infant chest wall leads to the well-known phenomenon of ‘paradoxical inward rib cage motion’ (PIRCM; also called thoraco-abdominal asynchrony) during the inspiratory phase of breathing. Multiple studies during the 1970s showed that the rib cage of otherwise normal infants collapses during the inspiratory descent of the diaphragm and is associated with deflation of the rib cage, independent of upper airway obstruction.11–13 The degree of thoraco-abdominal asynchrony is significantly greater in preterm versus full-term infants.
As expected, given the normal atonia that occurs during REMa sleep, PIRCM is more likely to occur during REM sleep. Even in full-term normal infants, PIRCM occurs during REM sleep and is associated with a lower and more variable PaO2.14 In mature, healthy, full-term infants with PIRCM during REM sleep, thoracic gas volume (TGV) was 31% reduced compared to TGV during NREM sleep.13 As noted above, such a large decrease in TGV during REM sleep markedly increases the probability of hypoxia with brief respiratory events, especially given that REM is the predominant sleep stage in infants, and O2 stores (primarily the lungs) are low relative to metabolic rate.9,10
At what age do normal infants stop exhibiting PIRCM during childhood? This is a key question for sleep medicine specialists, as PIRCM is considered a sign of increased upper airway resistance or obstruction in older children and adults. Gaultier and colleagues studied healthy infants between 7 and 31 months of age using polysomnography and diaphragmatic EMG during a daytime nap. The duration of PIRCM during sleep decreased as postnatal age increased.15 By 3 years of age, PIRCM is ‘rare or absent’ in normal children16 and does not occur during REM sleep in normal adolescents.17 Therefore, finding PIRCM in a child older than 3 years of age (with normal neuromuscular function) should raise suspicion for increased upper airway resistance or obstruction. However, it is important to note that the amount of measured ‘paradoxical breathing’ (PIRCM) might depend heavily on the technology used to detect it. In a study of 55 normal children 2–9 years of age without sleep-disordered breathing, PIRCM was detected in 40% of 30 s sleep epochs when piezo technology was used versus only 1.5% of epochs when respiratory inductance plethysmography (RIP) was used to detect thoraco-abdominal motion.18
Dynamic Maintenance of End Expiratory Lung Volume
Inhibition of respiratory muscle tone at any age results in a decrease in lung volume.19–22 In other words, lung volume is maintained, in part, by respiratory muscle activity. In full-term infants, during tidal breathing in NREM sleep, end-expiratory lung volume (EEV) is maintained above the passive relaxed lung volume (Vr).23 This is accomplished by multiple mechanisms including expiratory ‘braking’ using muscles of the upper airway, and post-inspiratory inspiratory activity (PIIA) of the diaphragm, which alter expiratory time constant-Te relationship such that expiration is terminated (interrupted) before reaching Vr.23–25
The strategy of maintaining EEV above Vr is sleep-state-dependent. Several studies of EEV in full-term infants were performed during behavioral NREM sleep but did not study the effects of sleep per se. When sleep state was studied, TGV was found to be greater in NREM sleep compared to REM sleep in full-term infants, suggesting that EEV was better maintained in NREM sleep.13 Preterm infants also maintain EEV above passive Vr, also with clear sleep-state dependency. Preterm infants ≈32 weeks’ gestational age were studied during the first week of life during REM and NREM sleep. In NREM sleep, a shortened expiratory time (TE) and diaphragmatic braking resulted in maintenance of EEV above Vr. In contrast, during REM sleep, Te was longer and expiratory braking was reduced such that EEV approached Vr.25
The dynamically maintained EEV, which helps maintain SpO2 in infants, may be lost during apnea. Preterm infants ≈29 weeks’ gestational age were studied during central apnea using intercostal muscle and diaphragm surface EMG activity as well as anterior–posterior (AP) diameter of the rib cage and abdomen (as a measure of EEV).21 During apnea, decreased activity of the respiratory muscles correlated with loss of EEV. The apnea-related drop in EEV was greater during NREM sleep, suggesting that EEV was better maintained during NREM compared to REM sleep.21 Thus, infants are able to compensate for their ‘mechanical disadvantage’ by maintaining EEV above passive Vr during sleep, although they do this less effectively during REM sleep. This has important clinical implications, given the importance of lung O2 stores in infants for maintaining normal SpO2. The loss of EEV in infants during apnea increases the probability of, and potentially the rapidity of, O2 desaturation.
How long does the active maintenance of EEV above Vr persist during infancy? In healthy infants and children aged 1 month to 8 years, studied using RIP-derived tidal breathing flow-volume loops to assess breathing strategy, the flow-volume pattern during expiration was ‘interrupted’ up to 6 months of age, consistent with dynamic maintenance of an elevated EEV during this period.26 Between 6 and 12 months, expiratory flow-volume patterns were a mixture of ‘interrupted’ and ‘uninterrupted,’ indicating a transitional period. After 1 year of age, expiratory flow-volume patterns were ‘uninterrupted,’ consistent with relaxed or passive end expiratory lung volume.26 Thus, the transition from dynamic maintenance of EEV to the mature, adult-like, passive or relaxed EEV occurs during the second half of the first year of life.
Relative Contribution of Rib Cage and Abdomen to Tidal Volume
Postnatal developmental changes in chest wall compliance and active maintenance of EEV predict that the relative contributions of the rib cage (RC) and abdomen (ABD) to tidal volume, and the effects of sleep, are likely to change with maturation. In studies of normal supine adults, the average rib cage contribution to Vt fell by 25–32% during REM sleep compared to waking, consistent with the normal skeletal muscle atonia that occurs during REM sleep.22,27 Similarly, in healthy term infants, the RC contribution to Vt was found to be lower in REM versus NREM sleep.28 As anticipated, based on normal maturation of chest wall compliance, the contribution of the rib cage to VT during NREM sleep (measured using RIP) increases during infancy between 1 and 26 months of age.29
Response to Mechanical Loading of the Respiratory System during Sleep
Elastic and resistive loads on the respiratory system are intrinsic to the normal lungs, chest wall and upper airway. In adults, nasal and upper airway resistance increases during normal sleep, although with considerable individual variation.30–32 Numerous disorders impose additional loading on the respiratory system, including lung disease and sleep-related upper airway obstruction, and the normal respiratory system responds with load compensation strategies that vary with type of load, age, position and state.
Resistive Loading
Awake, normal adults are able to compensate for added resistive loads and maintain minute ventilation (VE) and tidal volume (VT).33,34 This compensatory ability depends on the adequacy of the respiratory control system response as well as chest wall stability and respiratory muscle strength.33,35 During REM and NREM sleep in normal adults, progressive addition of inspiratory resistive loads decreases VE, largely due to inadequate prolongation of inspiratory time (TI) in the presence of increased inspiratory resistance.34
The much greater compliance of the chest wall during infancy, especially in preterm infants, predicts that infants may not cope well with inspiratory resistive loading. Preterm and full-term infants during NREM sleep exhibited similar lung resistance and compliance, although the preterm infants exhibited greater thoraco-abdominal asynchrony prior to loading compared to full-term.35 When presented with an inspiratory resistive load, full-term infants were able to maintain VE and VT with little effect on thoraco-abdominal synchrony. In contrast, identical inspiratory loading in preterm infants resulted in decreased VT and VE as well as increased chest wall asynchrony, suggesting that the preterm infant is less able to compensate due to chest wall instability.35
Resistive loading alters vagally mediated reflexes that modify mechanical and neural inspiratory duration. For example, in a study of full-term, 2–3-day-old infants during NREM sleep, application of increasing inspiratory resistive loads (to a single breath) resulted in progressive prolongation of TI, shortening of expiratory time (TE) and decreasing VT.36 These changes were also reflected in ‘neural’ VT, TI and TE as measured by EMG.36 In the same group of infants (during NREM sleep), increasing expiratory resistive loading was associated with progressive decrements in VE and prolongation of (neural and mechanical) TE, with little effect on TI.37 In summary, both inspiratory and expiratory resistive loading decrease minute ventilation in infants, but effects on breathing pattern differ. In addition, the effects of mechanical loading on respiratory timing in infants tend to be more pronounced with resistive loads versus elastic loads.36–38 Unfortunately, although studies were often performed during sleep, the effects of sleep per se were typically not examined.
Very few studies have reported the effects of respiratory mechanical loading in older normal children. Marcus and co-workers studied the effects of inspiratory resistive loading in normal children ≈9 years of age.39 When presented with a flow-resistive load for 3 minutes during sleep, in both REM and NREM sleep there was an immediate fall in VT and VE, which was proportional to the magnitude of the resistance and associated with a small increase in the ratio of TI to total breath time (TTOT) due to shortening of TE.39 A similar study of inspiratory resistive loading during NREM sleep in normal young adults (mean age 20.5 years) also reported a marked drop in VT (and VE), proportional to the magnitude of the resistive load.40 This was associated with significant prolongation of TI and increase of TI/TTOT, with no change in respiratory rate.40
Elastic Loading
Although there are multiple ways to impose an elastic load on the respiratory system, a common approach is to have the subject breathe from a closed volume reservoir (elastic load varies with the size of the reservoir). In normal adults, during wakefulness, application of an inspiratory elastic load is compensated immediately such that VT and VE are preserved.41 However, during NREM sleep, sustained inspiratory elastic loading caused a sustained drop in VT and VE until they were restored by increasing PCO2.41 Thus, during NREM sleep in adults, compensatory responses to elastic loading only occur when respiratory effort is increased by chemical stimuli.
In full-term, quietly sleeping infants, application of an elastic load during inspiration caused a marked fall in VT and prolongation of TI, with little effect on TE.36 Application of an elastic load during expiration in infants of the same age also caused a marked drop in VT and prolongation of TE, with little effect on TI.37 Similarly, in preterm infants ≈31 weeks’ gestational age and ≈8 days’ postnatal age, addition of external elastic loads caused a marked drop in VT and prolongation of TI and TE, the magnitude of which increased progressively with the magnitude of the elastic load.38
When application of respiratory elastic loading during sleep is prolonged in term and preterm infants, VT initially drops but progressively increases during subsequent breaths, indicating a compensatory response.12 In both term and preterm infants, load compensation was more effective during NREM sleep. During REM sleep, elastic loading increased rib cage distortion, which limited compensation.12 Increased respiratory elastic load occurs in numerous clinical conditions, including hyperinflation, obesity and neuromuscular disorders, and compensatory responses may be blunted by sleep, especially REM sleep.
Complete Airway Occlusion – Effects on Breathing during Sleep
Although review of the extensive literature on the Hering–Breuer (HB) and other lung-inflation reflexes is beyond the scope of this chapter, several points about the respiratory timing effects of total airway occlusion in children will be highlighted here. In 1868, Breuer and Hering described the role of the pulmonary slowly adapting stretch receptors (fibers carried in the vagus nerves) in determining the rate, depth and timing of tidal breathing.42 The HB reflexes are classically elicited in several ways; occlusion of the airway at the beginning of inspiration prolongs TI, while airway occlusion at end inspiration prolongs TE. The HB reflexes can be elicited in normal, unsedated adults, but volumes larger than the normal tidal volume range are required and evidence for the persistence of HB reflexes beyond infancy is controversial.43 In spite of multiple early studies suggesting that the HB reflexes participate in controlling tidal breathing during infancy, their role during postnatal development remains controversial.36,37,44,45
In full-term infants 2–3 days of age, during NREM sleep, total occlusion of the airway during inspiration (for a single breath) resulted in marked prolongation of mechanical and neural (EMG) TI, as expected due to removal of vagally mediated feedback from pulmonary slowly adapting stretch receptors, without effect on TE.36 In the same group of infants, total airway occlusion during expiration (for a single breath) led to marked prolongation of TE, without effect on TI.37 Beyond the newborn period, prolongation of TE by end-inspiratory occlusion persisted throughout the first year, although the magnitude of the TE prolongation decreased with age.46,47
Studies in preterm infants indicate that reflex effects of inspiratory airway occlusion on timing depend on gestational age at birth and chronological age. In one study of preterm infants, ≈30 weeks’ gestational age and ≈8 days old, the prolongation of TI was similar to that seen in term infants.48 Closer examination of the effects of maturity and age yielded different results. Preterm infants born at 27–32 weeks’ gestational age and studied within the first 3–4 days exhibited wide variation in the response to inspiratory occlusion.49 TI was prolonged in some and shortened in others, while the overall magnitude of prolongation was much less than the ≈30% prolongation of TI observed in full-term infants.49 In the same group of infants, the response to inspiratory occlusion was still immature at 7–10 days, but was similar to the full-term response by 14 days. In preterm infants born at 33–36 weeks’ gestational age, the TI prolongation with inspiratory occlusion was mature by 7–10 days, suggesting that the rapidity of maturation depends on the degree of maturity at birth.49
Sleep may profoundly affect Hering–Breuer reflexes in infants. In a study of preterm infants 30–36 weeks’ ‘postconceptual age’ (sic), using observational sleep staging, occlusion of a normal tidal breath at end inspiration resulted in an average TE prolongation of 87% in NREM sleep compared to 419% in REM sleep.45 However, another study, using single-breath inspiratory occlusions in term newborns, found increased Hering–Breuer activity in NREM compared to REM sleep.50
Caution should be exercised in the interpretation of studies using mechanical loading or total airway occlusion. As noted above, most studies only studied infants during NREM sleep. Many studies only examined the effects of occlusion for brief durations, even a single breath or parts of a breath. Reflex effects during longer occlusions may lead to increasing compensatory adaptations over time. In addition, these methods may evoke other reflex effects from face masks used and some authors have suggested that the upper airway negative pressure and other reflexes may contribute, potentially confounding interpretation.51
Maintenance of Upper Airway Patency during Sleep in Normal Children
The pharyngeal airway, extending from the nasal choanae to the epiglottis, is a collapsible tube composed of muscle and soft tissues, without support from bony structures except for the posterior pharyngeal wall.52 Pharyngeal patency depends on mechanical factors (muscle mass, connective tissue, submucosal fat, mucosal edema, perfusion, position, etc.) as well as activity of the muscles that compose and surround the airway.52,53 The pharyngeal muscles may act to stiffen the airway soft tissues, making them less deformable by intraluminal negative pressure, or actively dilate the airway and change its caliber.52
The pharyngeal airway is bounded by the posterior pharyngeal wall, anchored at the top by the palatal muscles (musculus uvulae, palatoglossus, palatopharyngeus, tensor veli palatine and levator veli palatine), at the bottom by the hyoid muscles (thyrohyoid, mylohyoid, stylohyoid, geniohyoid and sternohyoid) and anteriorly by the genioglossus muscle.52,54 The activity of these muscle groups is largely responsible for the maintenance of airway patency during sleep. It is important to note that, although most studies to date have focused on the genioglossus muscle, innervated by the hypoglossal nerve (XII), upper airway tone is influenced by input from other motoneuron groups including the motor vagus (X), glossopharyngeal (IX), facial (VII) and motor trigeminal (V).53 In addition, motoneurons of the cervical ventral horn may contribute to airway patency via their influence on neck and jaw position as well as lung volume.53 As these muscles are modulated by state, sleep may profoundly affect pharyngeal patency and reflex responses (e.g., to negative pressure).
A full review of normal upper airway structure, physiology and its development is beyond the scope of this chapter. The reader is referred to several excellent reviews of upper airway structural development during childhood.55–57 Here, we review what is known about the physiology of upper airway collapsibility in infants and children, how it can be measured, how airway patency is affected by sleep and the effects of puberty and age.
Critical Closing Pressure, Pcrit
The human upper airway behaves as a Starling resistor, modeled as a tube with rigid segments on either end and a collapsible segment in a sealed box in between (representing the collapsible pharynx and surrounding tissue mass).58 The patency of the collapsible segment is determined by the mechanical factors noted above and by the activity of upper airway muscles. Pcrit, the critical closing pressure of the collapsible segment, is the pharyngeal lumenal pressure when collapse occurs (Figure 23-2). In this model, the upstream (nasal) and downstream (hypopharyngeal/tracheal) segments have defined resistances and fixed diameters.
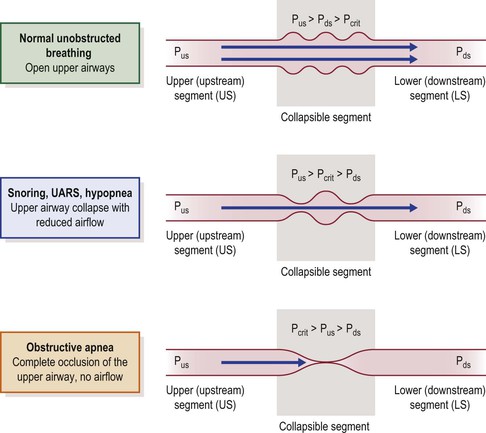
Figure 23-2 The upper airway modeled as a Starling resistor; airflow through a tube with rigid upstream (nasal airway) and downstream (hypopharyngeal airway) segments, and a collapsible segment in a sealed box in between (pharyngeal airway). The upstream and downstream segments have defined resistances and fixed diameters. Pus = pressure upstream. Pds = pressure downstream. The patency of the collapsible segment depends on the pressure exerted by the surrounding tissues (Pcrit). See text for explanation. From Kirkness JP, Krishnan V, Patil SP, Schneider H. Upper Airway Obstruction in Snoring and Upper Airway Resistance Syndrome. In: Randerath WJ, Sanner BM, Somers VK, eds. Sleep Apnea. Basel: Karger; 2006:79–89.
During inspiration, diaphragm contraction and thoracic cage expansion lower pressure in the downstream segment, creating a pressure gradient for inspiratory airflow. The degree of inspiratory airflow limitation (if any) depends on the pressure gradient between the upstream segment (Pus) and Pcrit, and is independent of downstream pressure (Pds). Pus at the nares is atmospheric pressure (zero cmH2O, reference) and Pcrit, in normals, is typically less than −10 cmH2O. Therefore, although there is a small pressure drop across the upstream segment, in normal subjects upstream pressure remains sufficiently greater than the pressure in the collapsible segment such that inspiratory airflow is unimpeded (Figure 23-2, upper panel).58,59
Any condition that increases Pcrit (upper airway dilator muscle hypotonia, sedation, anesthesia, obesity, edema) may reduce the difference between Pus and Pcrit, with resulting inspiratory airflow limitation (Figure 23-2, middle panel). When Pcrit exceeds Pus, complete obstruction occurs (Figure 23-2, lower panel).52,53,58,59 In simple terms, when pharyngeal critical closing pressure is positive to atmospheric pressure, complete airway collapse occurs during inspiration and upstream (nasal) positive pressure (greater than Pcrit) is required to restore pharyngeal patency (e.g., nasal continuous positive airway pressure (CPAP)). In normal infants, children and adults, Pcrit is negative during wakefulness and sleep, usually ≤10 cmH2O, and the pharyngeal airway is always patent, with no inspiratory airflow limitation.
Variations in Pcrit during wakefulness and sleep are due largely to its dependence on upper airway dilator muscle activity. During normal breathing, airway patency is heavily influenced by state-dependent activity of the upper airway dilator muscles. In addition, upper airway dilator muscles are activated by negative pressure in the airway, sensed by negative pressure receptors primarily in the larynx.52,60 Finally, the negative pressure reflex and respiratory drive to the upper airway dilator muscles are strongly influenced by the levels of chemical respiratory drive (PaO2 and PaCO2) from the peripheral and central chemoreceptors.61–65 All of these factors combine to make Pcrit dynamic, varying with position, sleep state, levels of chemical stimuli and other factors that affect pharyngeal deformability.
Measurement of Upper Airway Collapsibility
Upper airway collapsibility can be well-characterized by two values: (1) the slope of the linear relationship between maximal inspiratory flow (y-axis) and upstream (nasal) pressure (x-axis), and (2) Pcrit, the x-axis intercept (zero flow). The relationship between upper airway maximal inspiratory flow (VImax) and nasal pressure (PN) may be termed VImax/PN and is also termed ‘SPF’ (slope of the pressure–flow relationship) in the pediatric literature.66 Pcrit and VImax/PN can be determined experimentally in individual subjects to obtain numerical measures of upper airway collapsibility. Their classic laboratory measurement yields values that ‘characterize’ upper airway collapsibility during sleep for a particular sleep stage and specific method of measurement, in an individual subject. Pcrit and VImax/PN are typically measured during slow wave sleep and are difficult to measure during wakefulness or REM sleep.
In humans, Pcrit of the upper airway is typically measured during tidal breathing, using a nasal mask attached to source of negative or positive pressure. A pneumotachograph is used to measure inspiratory airflow and PN is measured at the mask.67,68 In normal subjects there is no inspiratory flow limitation when nasal pressure is zero (atmospheric pressure), so negative pressure is applied via the nasal mask and made more negative in steps to produce a VImax vs. PN relationship as shown in Figure 23-3. When maximal inspiratory airflow (for tidal breaths at a given pressure) is plotted versus nasal mask pressure, a linear relationship is obtained and extrapolated to zero flow. The slope of the VImax/PN relationship (aka ‘slope of the pressure–flow relationship’ (SPF) in the literature) represents the collapsibility of the upper airway and the x-axis (zero flow) intercept represents Pcrit (Figure 23-3). This approach has been used to characterize upper airway collapsibility in numerous studies of normal children69 and adults.67,68 In children with sleep-disordered breathing (SDB), when airflow limitation or obstructive apnea are already present at baseline (nasal mask pressure = 0), a linear pressure–flow relationship can be obtained in a similar manner by applying positive pressure via nasal mask, increasing in steps until airflow limitation is abolished.
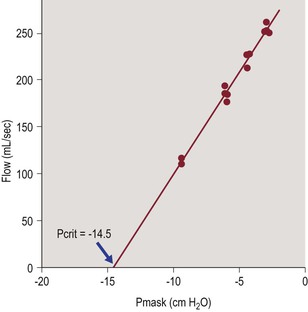
Figure 23-3 Typical measurement of pharyngeal critical closing pressure in a normal subject.
The slope of the pressure–flow relationship (in mL/sec/cmH2O) represents airway conductance and the x-axis intercept (zero flow) represents critical closing pressure (Pcrit). From Litman RS, McDonough JM, Marcus CL, Schwartz AR, Ward DS. Upper airway collapsibility in anesthetized children. Anesth Analg 2006;102:750–4.
Dynamic Upper Airway Negative Pressure Reflexes: Active Versus Passive Pcrit
In normal children during neuromuscular paralysis Pcrit is about −7.5 cmH2O,70 much higher than the Pcrit of normal children during sleep, which is about −25 cmH2O using the ‘gradual’ method of measurement (see below).66,71,72 Loads imposed on the upper airway that generate negative pressure in the pharynx and larynx may cause reflex activation of upper airway dilator muscles to stiffen and/or alter the caliber of the airway.52 This has very important implications for the measurement of the VImax/PN relationship and Pcrit. When upper airway collapsibility is measured in the classic way, by progressively stepping down nasal (mask) pressure, the upper airway dilator muscles are reflexly activated as nasal pressure is decreased (made more negative). Figure 23-4 shows a normal subject, in stage 2 NREM sleep, starting from a nasal mask holding pressure of 5 cmH2O.73 The left panel shows the ‘gradual’ approach, in which PN is decreased 1–2 cmH2O every 10 minutes, without returning to baseline holding pressure. This results in recruitment of compensatory upper airway dilator muscle activity, as evidenced by the marked genioglossus muscle EMG activity (Figure 23-4). The Pcrit and VImax/PN relationships measured using the ‘gradual’ approach include the effects of compensatory dilator muscle activity and are therefore also known as ‘active’ or ‘activated’ pressure–flow measurements.66
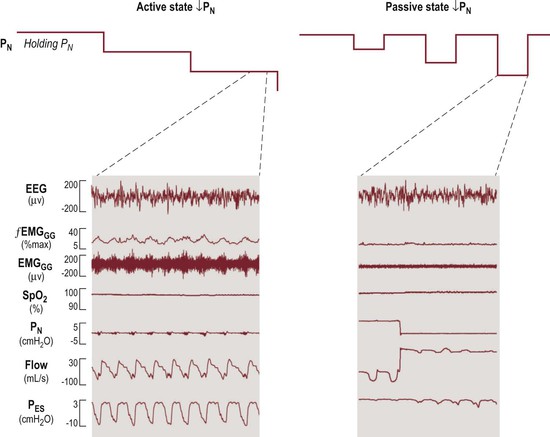
Figure 23-4 Comparison of the two methods of measuring upper airway collapsibility in a control subject. Left panel: ‘Gradual’ method. Left upper: From holding pressure of 5 cmH2O during stage 2 NREM sleep, nasal mask (PN) pressure is lowered in steps about every 10 minutes. Left lower: At −3 cmH2O nasal pressure, inspiratory flow limitation occurs and compensatory dilator muscle activity (EMGGG) is markedly increased. Right panel: ‘Intermittent’ method. Right upper: Starting from the same holding pressure (5 cm H2O) in stage 2 NREM sleep, nasal mask pressure is lowered intermittently for five breaths only and then returned to the baseline holding pressure. Right lower: At −3 cmH2O, in sharp contrast to the ‘gradual’ method (left), there is no compensatory upper airway dilator muscle activation. Modified from McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 2008;105:197–205.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


