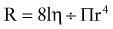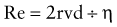Chapter 365 Respiratory Pathophysiology and Regulation
365.1 Lung Volumes and Capacities in Health and Disease
Ashok P. Sarnaik and Sabrina M. Heidemann
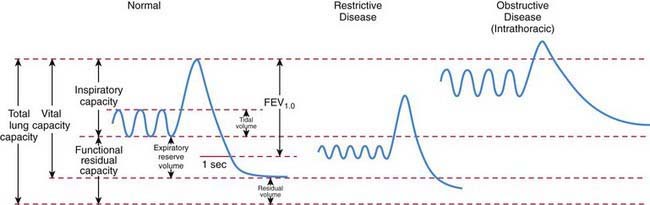
Traditionally, lung volumes are measured with a spirogram (Fig. 365-1). Tidal volume (VT) is the amount of air moved in and out of the lungs during each breath; at rest, tidal volume is normally 6-7 mL/kg body weight. Inspiratory capacity (IC) is the amount of air inspired by maximum inspiratory effort after tidal expiration. Expiratory reserve volume (ERV) is the amount of air exhaled by maximum expiratory effort after tidal expiration. The volume of gas remaining in the lungs after maximum expiration is residual volume (RV). Vital capacity (VC) is defined as the amount of air moved in and out of the lungs with maximum inspiration and expiration. VC, IC, and ERV are decreased in lung pathology but are also effort dependent. Total lung capacity (TLC) is the volume of gas occupying the lungs after maximum inhalation.
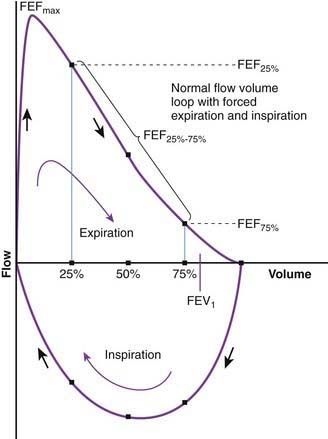
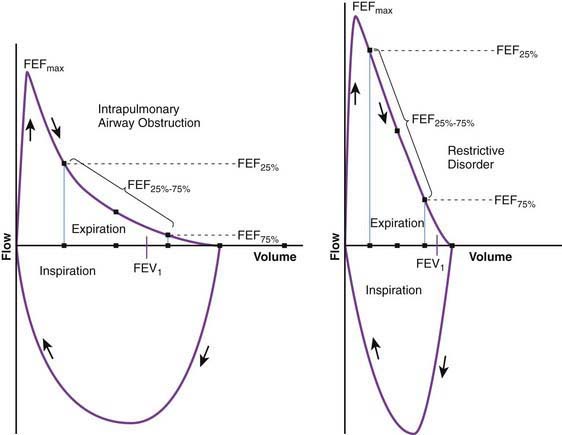
Flow volume relationship offers a valuable means at the bedside or in an office setting to detect abnormal pulmonary mechanics and response to therapy with relatively inexpensive and easy-to-use devices. After maximum inhalation, the patient forcefully exhales through a mouthpiece into the device until residual volume is reached followed by maximum inhalation (Fig. 365-2). Flow is plotted against volume. Maximum forced expiratory flow (FEF max) is generated in the early part of exhalation, and it is a commonly used indicator of airway obstruction in asthma and other obstructive lesions. Provided maximum pressure is generated consistently during exhalation, a decrease in flow is a reflection of increased airway resistance. The total volume exhaled during this maneuver is forced vital capacity (FVC). Volume exhaled in one second is referred to as FEV1. FEV1/FVC is expressed as a percentage of FVC. FEF25%-75% is the mean flow between 25% and 75% of FVC and is considered relatively effort independent. Individual values and shapes of flow-volume curves show characteristic changes in obstructive and restrictive respiratory disorders (Fig. 365-3). In intrapulmonary airway obstruction such as asthma or cystic fibrosis, there is a reduction of FEFmax, FEF25%-75%, FVC, and FEV1/FVC. Also, there is a characteristic concavity in the middle part of the expiratory curve. In restrictive lung disease such as interstitial pneumonia, FVC is decreased with relative preservation of airflow and FEV1/FVC. The flow volume curve assumes a vertically oblong shape compared to normal. Changes in shape of the flow volume loop and individual values depend on the type of disease and the extent of severity.
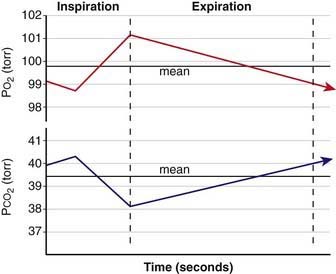
Functional residual capacity (FRC) is the amount of air left in the lungs after tidal expiration. FRC has important pathophysiologic implications. Alveolar gas composition changes during inspiration and expiration. Alveolar PO2 (PAO2) increases and alveolar PCO2 (PACO2) decreases during inspiration as fresh atmospheric gas enters the lungs. During exhalation, PAO2 decreases and PACO2 increases as pulmonary capillary blood continues to remove oxygen from and add CO2 into the alveoli (Fig. 365-4). FRC acts as a buffer, minimizing the changes in PAO2 and PACO2 during inspiration and expiration. FRC represents the environment available for pulmonary capillary blood for gas exchange at all times.
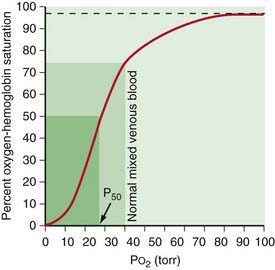
A decrease in FRC is often encountered in alveolar interstitial diseases and thoracic deformities. The major pathophysiologic consequence of decreased FRC is hypoxemia. Reduced FRC results in a sharp decline in PAO2 during exhalation because a limited volume is available for gas exchange. PO2 of pulmonary capillary blood therefore falls excessively during exhalation, leading to a decline in arterial PO2 (PaO2). Any increase in PAO2 (and therefore PaO2) during inspiration cannot compensate for the decreased PaO2 during expiration. The explanation for this lies in the shape of O2-hemoglobin (Hb) dissociation curve, which is sigmoid shaped (Fig. 365-5). Because most of the oxygen in blood is combined with Hb, it is the percentage of oxyhemoglobin (SO2) that gets averaged rather than the PO2. Although an increase in arterial PO2 cannot increase O2-Hb saturation >100%, there is a steep desaturation of hemoglobin below a PO2 of 50 torr; thus, decreased SO2 during exhalation as a result of low FRC leads to overall arterial desaturation and hypoxemia. The adverse pathophysiologic consequences of decreased FRC are ameliorated by application of positive end expiratory pressure (PEEP) and increasing the inspiratory time during mechanical ventilation.
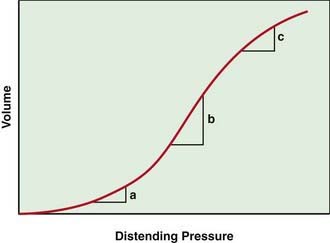
The lung pressure–volume relationship is markedly influenced by FRC (Fig. 365-6). Pulmonary compliance is decreased at abnormally low or high FRC.
365.2 Chest Wall
Ashok P. Sarnaik and Sabrina M. Heidemann
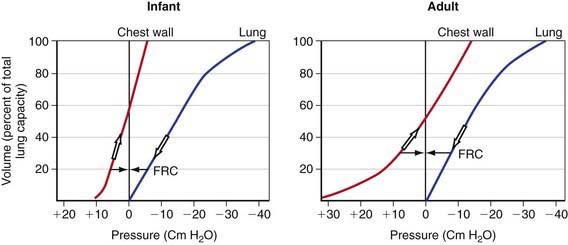
The chest wall and diaphragm of an infant are mechanically disadvantaged compared to that of an adult when required to increase thoracic (and therefore the lung) volume. The infant’s ribs are oriented much more horizontally and the diaphragm is flatter and less domed. The infant is therefore unable to duplicate the efficiency of upward and outward movement of obliquely oriented ribs and downward displacement of the domed diaphragm in an adult to expand the thoracic capacity. Additionally, the infant’s rib cage is softer and thus more compliant compared to an adult’s. Although a soft, highly compliant chest wall is beneficial to a baby in its passage through the birth canal and allows future lung growth, it places the young infant in a vulnerable situation under certain pathologic conditions. Chest wall compliance is a major determinant of FRC. Because the chest wall and the lungs recoil in opposite directions at rest, FRC is reached at the point where the outward elastic recoil of the thoracic cage counterbalances the inward lung recoil. This balance is attained at a lower lung volume in a young infant because of the extremely high thoracic compliance compared to older children (see Fig. 365-7 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). The measured FRC in infants is higher than expected because respiratory muscles of infants maintain the thoracic cage in an inspiratory position at all times. Additionally, some amount of air trapping during expiration occurs in young infants.
Abnormalities of the chest wall are encountered in certain pathologic conditions. Chest wall instability can result from trauma (fractured ribs, thoracotomy) and neuromuscular diseases that lead to intercostal and diaphragmatic muscle weakness. The increased chest wall compliance makes such children more vulnerable to respiratory decompensation when faced with similar pulmonary pathology compared to older children and adults with stiffer chest walls. Children with rigid, noncompliant chest wall (asphyxiating thoracic dystrophy of Jeune [Chapters 411.3 and 691], achondroplasia [Chapter 411.4]) have markedly diminished lung volumes and capacities.
365.3 Pulmonary Mechanics and Work of Breathing in Health and Disease
Ashok P. Sarnaik and Sabrina M. Heidemann
where R is resistance, l is length, η is viscosity, and r is the radius. The practical implication of pressure-flow relationship is that airway resistance is inversely proportional to its radius raised to the 4th power. If the airway lumen is decreased in half, the resistance increases 16-fold. Newborns and young infants with their inherently smaller airways are especially prone to marked increase in airway resistance from inflamed tissues and secretions. In diseases in which airway resistance is increased, flow often becomes turbulent. Turbulence depends to a great extent on the Reynolds number (Re), a dimensionless entity, which is calculated as
where r is radius, v is velocity, d is density, and η is viscosity. Turbulence in gas flow is most likely to occur when Re exceeds 2000. Resistance to turbulent flow is greatly influenced by density. A low-density gas such as helium-oxygen mixture decreases turbulence in obstructive airway diseases such as viral laryngotracheobronchitis and asthma. Neonates and young infants are predominantly nose breathers and therefore even a minimal amount of nasal obstruction is poorly tolerated.
365.4 Airway Dynamics in Health and Disease
Ashok P. Sarnaik and Sabrina M. Heidemann
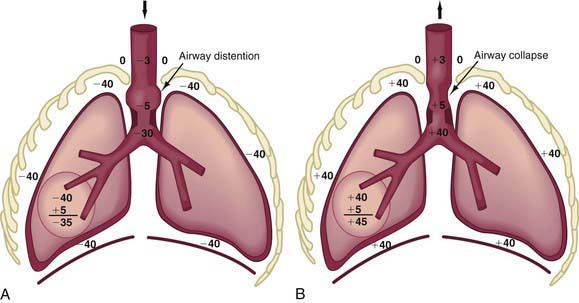
In extrathoracic airway obstruction (choanal atresia [Chapter 368], retropharyngeal abscess, laryngotracheobronchitis [Chapter 377]), the high negative intrapleural pressure during inspiration is transmitted up to the site of obstruction, after which there is a rapid dissipation of pressure. Therefore, the extrathoracic airway below the site of obstruction has markedly increased negative pressure inside, resulting in its collapse, which makes the obstruction worse (Fig. 365-8A). This produces inspiratory difficulty, prolongation of inspiration, and inspiratory stridor. Also, the increased negative intrapleural pressure results in chest wall retractions. During expiration, the increased positive intrapleural pressure is again transmitted up the airways to the site of obstruction, leading to a distention of the extrathoracic airway and amelioration of obstruction (Fig. 365-8B).
In obstruction of intrathoracic-extrapulmonary airway (vascular ring [Chapter 378], mediastinal tumors) and intrapulmonary airway (asthma, bronchiolitis), the increased negative intrapleural pressure results in a distention of intrathoracic airways during inspiration, thus providing some relief from obstruction (Fig. 365-9A).