Respiratory Distress Syndrome
Gross Ian
Respiratory distress syndrome (RDS) of the newborn, or hyaline membrane disease, is a major cause of illness and death in premature infants. It is the result of immaturity of the lungs at birth and, therefore, with rare exceptions, is seen only in premature infants. Although alveoli first appear at 28 weeks’ gestation, lung maturation is usually not adequate to sustain extrauterine life without some form of respiratory support until 32 to 34 weeks’ gestation. Babies born earlier than this may have inadequate surfactant, which results in decreased compliance of the lung. Other factors that contribute to lung compliance, such as tissue elasticity, are also believed to be abnormal in these infants. In addition, the anatomic structure of the lung in these infants is not as well suited to gas exchange as that of full-term infants because of the presence of smaller alveoli with larger amounts of interstitial tissue between them. The net result is a lung that is stiff and less well adapted for gas exchange.
EPIDEMIOLOGY
The incidence of RDS varies from country to country throughout the world. It depends on the gestational age of the newborn infant and whether the infant’s mother received antenatal glucocorticoid treatment. In general, the incidence of RDS in infants born before 30 weeks’ gestation is approximately 60% in those who have not been exposed to antenatal glucocorticoids and about 35% in those who have received an adequate course of glucocorticoid therapy. Between 30 and 34 weeks’ gestation, the incidence is approximately 25% in untreated or inadequately glucocorticoid-treated infants, and approximately 10% in those who have received a full course of treatment. In premature infants of more than 34 weeks’ gestational age, the incidence is approximately 5%, and RDS is rare in full-term infants.
FACTORS MODIFYING THE RISK OF RESPIRATORY DISTRESS SYNDROME OF THE NEWBORN
The factors associated with an increased risk of RDS include prematurity, maternal diabetes (classes A to C), delivery by cesarean section without antecedent labor, perinatal asphyxia, second twin, and history of a previous infant with RDS. The increased incidence of RDS in infants of diabetic mothers may be related to the hyperglycemia, increased ketones, or hyperinsulinemia that occurs during fetal life. Because labor enhances surfactant production, infants born by cesarean section that is not preceded by labor have an increased incidence of RDS. Acute asphyxia with hypoxia and acidosis appears to inhibit surfactant production. The incidence of RDS is higher in a second-born twin; whether this finding is related to asphyxia is not clear. Finally, there appears to be a familial tendency toward RDS, and a history of a sibling with RDS places a subsequent premature infant at higher risk.
Also, certain conditions decrease the incidence of RDS, such as long-term maternal stress (e.g., toxemia, hypertension), intrauterine growth restriction, maternal infection, maternal heroin exposure, and glucocorticoid treatment. Chronic low-grade maternal stress, as opposed to acute asphyxia, accelerates lung maturation by a mechanism that is not entirely clear. It is possible that hormones such as glucocorticoids are involved.
PATHOGENESIS
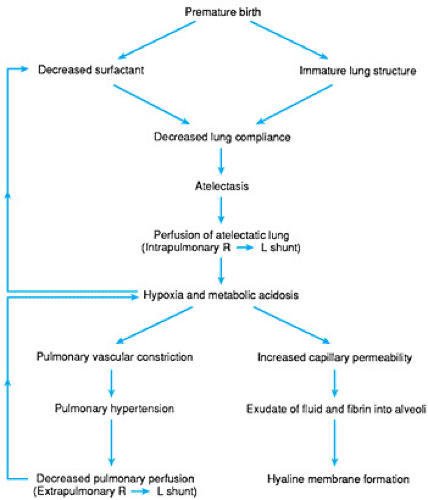
Current concepts of the pathogenesis of RDS are illustrated in Figure 42.1. The basic deficit is immaturity of surfactant production and lung structure. This results in a lung that is stiff and prone to atelectasis. Blood continues to perfuse the poorly ventilated areas, resulting in a ventilation-perfusion mismatch hypoxemia. The hypoxemia may in turn cause metabolic acidosis as a result of poor tissue oxygenation. In the presence of hypoxemia, the pulmonary arterioles tend to constrict, producing increased pressure in the pulmonary circulation. Shunting of blood from the right atrium to the left atrium through the foramen ovale may now occur, further aggravating the hypoxemia. Depending on the relative pressures in the pulmonary artery and the aorta, there also may be right-to-left shunting through a patent ductus arteriosus. In most instances, this process is reversible if normal blood gas levels are restored.
Alveolar disruption and necrosis can also occur, resulting in leakage of fluid and fibrin from the pulmonary capillaries into the alveolar space and small airways. This fluid and fibrin
exudate eventually coalesces to form proteinaceous deposits that, at postmortem examination, are identified as the eosinophilic hyaline membranes characteristic of this disease. It is clear that hyaline membrane formation is a consequence and not a cause of RDS. Hyaline membranes have never been described in stillborn infants; they appear only in infants who have been alive and sick for at least a few hours.
exudate eventually coalesces to form proteinaceous deposits that, at postmortem examination, are identified as the eosinophilic hyaline membranes characteristic of this disease. It is clear that hyaline membrane formation is a consequence and not a cause of RDS. Hyaline membranes have never been described in stillborn infants; they appear only in infants who have been alive and sick for at least a few hours.
The ductus arteriosus may also play a role in the development of this disorder. If it is patent, as it usually is during the first day after birth in premature infants, blood may flow from right to left, as discussed earlier. If aortic pressure exceeds pulmonary artery pressure, however, flow will be from left to right and overperfusion of the lungs with pulmonary edema may ensue. This complication will further decrease lung compliance.
Finally, because surfactant production is decreased by hypoxia and acidosis, a cycle may be initiated whereby the infant starts off with inadequate surfactant production and becomes hypoxic and acidotic. This, in turn, further inhibits the production of surfactant.
CLINICAL MANIFESTATIONS
The clinical course of uncomplicated RDS usually follows a fairly consistent pattern. The infant demonstrates signs of respiratory distress that become worse during the first few hours after birth. In some infants, especially those who are extremely immature, the respiratory distress may be severe from the start. The disease worsens for 48 to 72 hours, reaches a peak, and starts to improve. The onset of recovery is often associated with diuresis. This classic course may not occur, however, in infants with very low birth weights or those who are very sick. With the use of ventilators and high oxygen concentrations, oxidant or mechanical lung injury may induce secondary lung damage, and a prolonged respiratory illness may ensue.
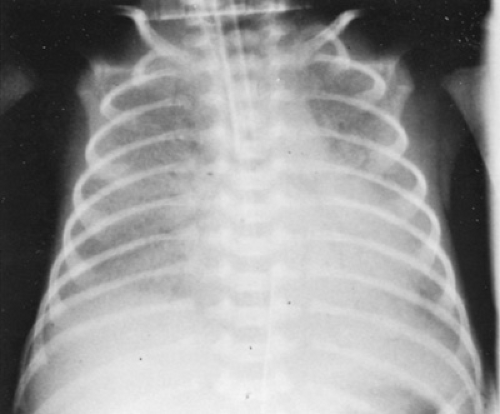
Infants with RDS initially have the classic features of respiratory distress in the newborn (i.e., tachypnea, flaring of the nose, retractions of the chest, cyanosis, and grunting). Radiographs taken at approximately 6 hours after birth reveal evidence of diffuse atelectasis and loss of lung volume (Fig. 42.2). The lung fields, which are relatively opaque, have been described as resembling ground glass or being reticulogranular. Because the lung fields are radiodense, the heart border may be obscured. In addition, air within the bronchi stands out in contrast to the lung fields as “air bronchograms” that may extend down to the diaphragm. This radiographic picture, although characteristic of RDS, may also be seen in neonatal pneumonia in premature infants, and it may be impossible to distinguish RDS from pneumonia on radiographic grounds.
If the infant dies, the characteristic features at postmortem examination are disrupted alveoli and the presence of eosinophilic hyaline membranes. Although these proteinaceous deposits give this syndrome its name, the presence of hyaline membranes is not pathognomonic of “hyaline membrane disease.” They also are seen in pneumonia, cardiac failure, and other neonatal conditions characterized by lung injury.
The differential diagnosis of RDS includes other causes of respiratory distress in the premature infant. The condition with which it is most likely to be confused is group B streptococcal pneumonia, which may mimic RDS in almost every respect. At times, it may be possible to differentiate these two disorders only retrospectively, by reviewing the course and pattern of the illness.
COMPLICATIONS
The major complications of RDS are related to therapy. Air leak is caused by increased airway pressure from mechanical ventilation or continuous positive airway pressure (CPAP). This complication is discussed in more detail in Chapter 46, Pulmonary Air Leaks. Chronic lung disease (bronchopulmonary dysplasia) is believed to result from injury to the lungs by oxygen and ventilation. The fragile lung of the extremely premature infant is particularly susceptible to injury, so the incidence of bronchopulmonary dysplasia is greatly increased in infants with birth weights of less than 1,250 g. Bronchopulmonary dysplasia is reviewed in Chapter 50, Bronchopulmonary Dysplasia.
Catheter complications arise from the insertion of a catheter into the aorta, which can result in thromboembolic complications such as hypertension due to renal arterial thrombosis. Additionally, an increased incidence of intraventricular hemorrhage is seen in infants with RDS. The mechanism may be related to intravascular pressure swings. Because premature infants with RDS are treated with oxygen, they are at particular risk for retinopathy of prematurity. Like bronchopulmonary dysplasia, this complication occurs mainly in infants with birth weights of less than 1,250 g.
Catheter complications arise from the insertion of a catheter into the aorta, which can result in thromboembolic complications such as hypertension due to renal arterial thrombosis. Additionally, an increased incidence of intraventricular hemorrhage is seen in infants with RDS. The mechanism may be related to intravascular pressure swings. Because premature infants with RDS are treated with oxygen, they are at particular risk for retinopathy of prematurity. Like bronchopulmonary dysplasia, this complication occurs mainly in infants with birth weights of less than 1,250 g.
THERAPY
Respiratory Care
A major role of neonatal intensive care units is providing respiratory care to infants with RDS. The level of intervention required depends on the severity of the respiratory distress. This is determined primarily by the arterial blood gas levels and by the amount of supplemental oxygen that the infant needs to maintain an adequate arterial oxygen tension (PaO2). Generally, the goal of therapy is to keep the PaO2 in the range of 45 to 70 mm Hg, the PaCO2 between about 45 and 55 mm Hg, and the pH above 7.20.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree