Chapter 53
Respiratory Disease
Karen S. Lindeman MD
Chapter Outline
Asthma
Definition
Asthma is defined by the presence of the following three characteristic findings: (1) reversible airway obstruction, (2) airway inflammation, and (3) airway hyperresponsiveness. Airway obstruction produces the clinical manifestations of wheezing, cough, and dyspnea. Airway inflammation modulates the course of asthma by independently producing airway obstruction and enhancing airway hyperresponsiveness. Airway hyperresponsiveness is marked by exaggerated responses to a wide variety of bronchoconstrictor stimuli, including histamine, methacholine, prostaglandin F2α, hypo-osmotic solutions, and cold air.
Epidemiology
Asthma is an increasingly common problem among young, otherwise healthy women of childbearing age. Morbidity and mortality rates from this disease increased during the 1980s and 1990s. From 2001 to 2010, the prevalence of asthma in the United States increased from 7.3% to 8.4%.1
The prevalence of asthma in women of childbearing age also continues to rise. The rate was approximately 3% in the 1990s and has increased to approximately 8.8% in the early 2000s.2
Pathophysiology
Asthma is believed to occur under a variety of environmental influences in the presence of genetic susceptibility.3 The underlying defect that produces the clinical syndrome of asthma is unknown. The most important potential mechanisms are (1) an enhancement of contractility or an impairment of relaxation of airway smooth muscle, (2) a neural imbalance, (3) airway inflammation, and (4) changes in the function of the airway epithelium.
Airway Smooth Muscle
Contraction of airway smooth muscle is believed to be the most important factor in producing acute airway obstruction. For many years, an enhancement of airway smooth muscle responsiveness to contractile agonists was assumed to be a major mechanism of asthma. To test this hypothesis, investigators attempted to correlate airway responsiveness in vivo and in vitro in humans4–8 and in the basenji-greyhound dog model of asthma.9 These studies did not demonstrate a significant correlation between the airway response to histamine or cholinergic agonists in vivo and airway smooth muscle contraction in vitro. Some studies actually demonstrated a negative correlation between the in vivo and in vitro responses,8,9 suggesting that diminished responsiveness may represent a chronic adaptive response of airway smooth muscle.
Instead of an enhancement in responsiveness to contractile stimuli, a reduction in responsiveness to relaxant stimuli may contribute to airway obstruction. One study demonstrated impaired relaxant responses to isoproterenol in airway smooth muscle from human asthmatic subjects in comparison with the responsiveness of airway smooth muscle from controls.10 Other evidence substantiates the presence of impaired airway relaxation in asthmatic subjects in vivo.11 Although the mechanism for this effect is poorly understood, a reduction in airway sensitivity to beta-adrenergic agonists could contribute to airway hyperresponsiveness by altering the balance between constricting and dilating influences.
Neural Components
A balance between constricting and dilating influences also exists with respect to the autonomic nervous system. A shift in this balance, with an increase in constricting influences, may be a mechanism of asthma.
The parasympathetic nervous system provides the dominant constrictor input to the airways (Figure 53-1). Efferent cholinergic fibers travel in the vagus nerve to synapse in ganglia within the airway wall.12 Postganglionic fibers release acetylcholine to activate muscarinic receptors and stimulate airway smooth muscle contraction. A negative feedback system limits release of acetylcholine from nerve terminals. Muscarinic autoreceptors, or receptors on the nerve ending,13 also are activated by acetylcholine and inhibit further release of acetylcholine from the nerve terminal.
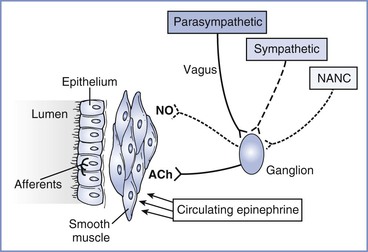
FIGURE 53-1 Neural control of the airway. Parasympathetic, sympathetic, and nonadrenergic noncholinergic (NANC) efferents innervate ganglia within the airway wall. Postganglionic cholinergic efferents release acetylcholine (ACh) to constrict airway smooth muscle. Postganglionic NANC efferents release nitric oxide (NO) to relax airway smooth muscle. Circulating epinephrine relaxes the airway. Afferents from the airway originate in the epithelium and are activated by airway irritation, as occurs with tracheal intubation.
The importance of exaggerated cholinergic efferent activity in the pathogenesis of airway hyperreactivity has been debated extensively. The relatively limited efficacy of anticholinergic agents in relieving clinical bronchospasm, as well as growing evidence supporting other mechanisms, suggests that this pathway has a limited role in the pathophysiology of asthma. However, this mechanism appears to be very important in the perioperative management of asthmatic subjects. Reflex stimulation of airway smooth muscle by placement of a tracheal tube represents one of the most important causes of bronchospasm in the perioperative period.
An alternative mechanism by which the parasympathetic nervous system may contribute to airway hyperresponsiveness is through dysfunction of the muscarinic autoreceptors. Dysfunction of these receptors allows increased postganglionic release of acetylcholine after reflex stimulation.14 This mechanism is well established in a guinea pig model of viral infection15 and may explain the airway hyperresponsiveness that occurs for several weeks after an upper respiratory tract infection, although additional autoreceptor-independent mechanisms may also be present.16 The role of this mechanism in the pathophysiology of clinical asthma is unclear.
The sympathetic nervous system primarily acts to decrease airway tone. In contrast to the parasympathetic nervous system, sympathetic innervation of airway smooth muscle in human subjects is either sparse or absent.17 Circulating catecholamines activate beta-adrenergic receptors in airway smooth muscle and provide the primary sympathetic efferent input to human airways. Because airways of normal human subjects do not become hyperresponsive after beta-adrenergic blockade,18 it is unlikely that impaired catecholamine secretion contributes significantly to the pathogenesis of asthma.
The alpha-adrenergic system is thought to play a relatively minor role in determining the state of airway responsiveness. Although alpha-adrenergic receptors are present in human airways,19 the protective effects of alpha-adrenergic antagonists have been disappointing and can be attributed to other properties, such as antihistamine activity.
In addition to cholinergic and adrenergic input, a third neural system, the nonadrenergic noncholinergic (NANC) system, provides efferent nerves to the airways. Both constricting and dilating pathways have been identified.20 Nitric oxide serves as the inhibitory NANC neurotransmitter in human airways.21 Potentially, a relative increase in constricting influences or a decrease in dilating influences in the NANC system could contribute to asthma. However, asthmatic subjects demonstrated no deficit in NANC inhibitory pathways,22 and inhibition of NANC excitatory neurotransmission did not improve airway hyperresponsiveness.23 Thus, current evidence does not support imbalance of the NANC system as a major mechanism of asthma.
Airway Inflammation
Airway inflammation appears to serve primarily as a modulating influence in asthma. Inflammation is certainly present in some but not all asthmatic subjects.24 The process of inflammation involves the occurrence of airway wall edema and infiltration of the mucosa by a variety of inflammatory cells, including neutrophils, mast cells, helper T lymphocytes, macrophages, and eosinophils.25 These cells produce and release mediators of inflammation, such as histamine, leukotrienes, platelet-activating factor, prostaglandins, thromboxanes, cytokines, serotonin, and nitric oxide.25 Mediators can modulate airway responsiveness by stimulating airway smooth muscle contraction,26 directing migration of inflammatory cells,27 modifying neural control of the airways,28 increasing mucosal permeability,29 or disrupting airway epithelium.30 In addition, airway inflammation can reduce airway diameter. Airway hyperresponsiveness is correlated with increased baseline airway tone.31 The overall importance of inflammation in asthma has been debated. Although inflammation appears to modulate the course of asthma, other factors certainly contribute to the pathogenesis.
Airway Epithelium
The epithelium provides a barrier to protect the subepithelial layers against stimuli that could provoke bronchospasm. Airways of asthmatic subjects demonstrate areas of epithelial destruction,32 and the clinical significance of this finding has been demonstrated.33
The epithelium not only serves as a barrier but also plays an active role in the maintenance of airway tone. The epithelium produces constricting and dilating factors.34,35 An alteration in the balance between these factors could alter airway responsiveness. The relative importance of alterations in epithelial function in the pathogenesis of asthma is unknown.
Diagnosis
Medical History
The classic symptoms of asthma include wheezing, cough, dyspnea, and chest tightness. A patient’s medical history also should include information about the pattern and severity of the symptoms, precipitating and aggravating factors, and the duration and course of these symptoms.
Physical Examination
Physical examination is directed to the respiratory tract. Auscultation of the chest may reveal wheezing and a prolonged phase of expiration.
Laboratory Studies
Laboratory studies that aid in the diagnosis of asthma depend on findings from the medical history and physical examination. In general, pulmonary function tests are useful to document the severity and establish the reversibility of obstruction (Box 53-1). In the absence of additional findings, other tests are not as useful in establishing the diagnosis of asthma. Bronchoprovocation tests (with agents such as methacholine or histamine) are used when the history and physical examination strongly suggest the presence of asthma but spirometry does not show airway obstruction.
Interaction with Pregnancy
Effects of Pregnancy on Asthma
The overall course of asthma has been reported to improve, worsen, or remain the same during pregnancy.36 Earlier evidence suggested that patients with more severe asthma are more likely to experience deterioration during pregnancy,36 but a recent study demonstrated that asthma severity during pregnancy is similar to severity during the year before pregnancy, provided that patients continue to use their prescribed medication during pregnancy. The investigators concluded that even mild asthma is likely to become significantly more severe if women discontinue their prescribed medication during pregnancy.37 A likely reason for the variation in the results of published studies is the difference in methods of assessing the severity of asthma. Most studies have used either clinical symptoms or requirements for pharmacologic therapy as indicators of the course of the disease. These measures do not correlate with objective measures of airway obstruction.38 Juniper et al.39 measured methacholine sensitivity before, during, and after pregnancy. Measurements of sensitivity to methacholine made during the second and third trimesters were lower than preconception or postpartum measurements (Figure 53-2). Although these findings suggest a reduction in airway hyperresponsiveness during pregnancy, the limited study population (16 subjects) makes extrapolation of the data to the general population unclear. Exacerbations of asthma during labor and delivery occur in as many as 20% of subjects37 and occur more frequently after cesarean delivery than after vaginal delivery (41% and 4%, respectively).40
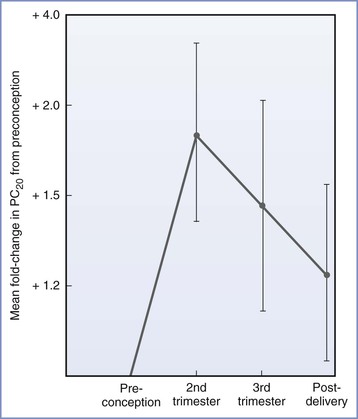
FIGURE 53-2 Airway responsiveness before, during, and after pregnancy expressed as fold change in PC20—dose of methacholine needed to reduce FEV1 (forced expiratory volume in 1 second) by 20%—compared with values before conception (n = 16; P = .033 for the effect of pregnancy on airway responsiveness). (From Juniper EF, Daniel EE, Roberts RS, et al. Improvement in airway responsiveness and asthma severity during pregnancy. Am Rev Respir Dis 1989; 140:924-31.)
A number of mechanisms may be responsible for the changes in the clinical course of asthma during pregnancy (Box 53-2). An increase in the progesterone level is thought to be one mechanism that improves asthma during pregnancy. Progesterone relaxes uterine and gastrointestinal smooth muscle and may or may not have similar effects on airway smooth muscle. However, Juniper et al.39 did not demonstrate a strong association between methacholine responsiveness and progesterone levels during pregnancy, suggesting that progesterone does not play a central role in attenuating airway hyperresponsiveness. In contrast, progesterone may actually worsen asthma by enhancing inflammation.41 Thus, effects of pregnancy on asthma appear to involve a number of factors other than direct effects of hormones on airway smooth muscle.
Effects of Asthma on the Parturient and Fetus
Many investigators have questioned whether maternal asthma adversely affects perinatal outcome. Differences in study design (e.g., retrospective, prospective) and differences in severity and treatment of asthma may account for different study results. Some studies have reported an increased incidence of preeclampsia,42,43 cesarean delivery,44–46 low-birth-weight (LBW) infants,47 preterm labor,45,48 antepartum and postpartum hemorrhage,49 and perinatal mortality.50 Diabetes mellitus appears to be more common among asthmatic patients treated with corticosteroids.51 Severe or poorly controlled asthma is a predictor of adverse outcome.40 Although asthma in pregnancy is associated with an increased risk for adverse perinatal outcomes, a meta-analysis of cohort studies suggested that active asthma management, which is intended to reduce the exacerbation rate, may reduce the risk for perinatal complications, particularly preterm delivery.52 No controlled studies have documented better perinatal outcome with aggressive asthma treatment. Potential mechanisms of increased perinatal morbidity and mortality in patients with uncontrolled asthma include hypoxemia and hypocapnia, inflammation, and altered placental function from asthma-associated mediator release.53 Siddiqui et al.54 have documented an association between preeclampsia and airway hyperresponsiveness and have proposed that the mechanism involves an interaction between mast cells and smooth muscle. A large prospective study is needed to confirm this association.
Medical Management
Pharmacologic therapy for asthma during pregnancy is directed toward avoiding acute exacerbations and episodes of status asthmaticus. Ideally, management should begin before conception. Although general principles typically dictate that unnecessary medication should be avoided during pregnancy, studies investigating the effects of asthma on perinatal outcome suggest that the risks for uncontrolled asthma are significantly higher than medication-associated risks.55 Medications that are currently used to treat asthma fall into two general categories: bronchodilators and anti-inflammatory agents. These agents generally are safe for the fetus. The prophylactic use of antibiotics is unnecessary.
Bronchodilators
Beta-adrenergic agonists exert beneficial effects in asthmatic patients by activation of β2-adrenergic receptors, which mediate a number of processes (Box 53-3). Short-acting beta-adrenergic agonists represent the most effective therapy for acute exacerbations of asthma.56 Daily use of long-acting beta-adrenergic agonists is controversial. Long-acting beta-adrenergic agonist therapy is associated with a significant increase in the risk for death,57 but controlled studies have not confirmed a cause-and-effect relationship.58 Certain genetic polymorphisms affect responses to short-acting but not long-acting beta-adrenergic agonists,59 leading to hopes that a personalized approach to therapy would improve clinical efficacy. Although regular use of beta-adrenergic agonists in asthma may be beneficial in conjunction with other forms of therapy, these agents do not appear to provide optimal control when used alone. Conversely, no compelling evidence requires that beta-adrenergic agonists be discontinued after conception or that their use be reserved for treatment of an acute exacerbation.
These agents may be administered as aerosols, orally, or parenterally. The aerosol route is generally preferred during pregnancy because high concentrations of the medication can be delivered directly to the site of activity in the airways, with relatively less drug delivered to the uteroplacental circulation.
The limited number of human studies investigating the fetal safety of long-term administration of a beta-adrenergic agonist have not shown significant adverse neonatal outcomes.60,61 In addition, the long history of use of these agents without reports of teratogenicity suggests that their use should not be restricted because of fetal concerns. Optimal control of maternal symptoms of asthma appears to be more important for the fetus than potential detrimental effects of beta-adrenergic agonists.
On the basis of the potential risks of long-term single-agent therapy with a beta-adrenergic agonist, a paradoxical approach to the treatment of asthma may involve long-term administration of a beta-adrenergic antagonist.62 This approach is analogous to the paradigm based in the cardiovascular system, in which long-term administration of a beta-adrenergic antagonist is beneficial in patients with congestive heart failure. Studies in asthmatic patients are ongoing.
Methylxanthines (e.g., theophylline, aminophylline) were used for many years in the long-term treatment of asthma. Although their mechanism of action is controversial, relaxation of airway smooth muscle is the most prominent effect. The ability of the agents to inhibit intracellular phosphodiesterase and increase concentrations of cyclic adenosine monophosphate (cAMP) is not the mechanism of bronchodilation, because these effects do not occur at clinically relevant concentrations in vivo.63 Furthermore, in the patient taking anti-inflammatory agents and beta-adrenergic agonists, methylxanthines add little to optimal asthmatic control.64 Although their use is now limited to patients whose asthma responds poorly to other forms of therapy, methylxanthines do not appear to cause significant adverse fetal outcomes.61 Serum concentrations of theophylline should be monitored carefully, especially in the third trimester, when theophylline clearance decreases.65
Bronchodilation with anticholinergic agents occurs through the blockade of muscarinic receptors on airway smooth muscle. Overall, anticholinergic agents alone are not as effective as beta-adrenergic agonists, but some patients show better response to anticholinergic agents.66 The effects of adding anticholinergic agents to beta-adrenergic agonists for acute67 and chronic68 asthma were evaluated in meta-analyses of randomized trials. Anticholinergic agents improved lung function in acute asthma67 but had little benefit in chronic asthma.68 The quaternary anticholinergic agent ipratropium bromide can be delivered as an aerosol, allowing higher concentrations in the lung with reduced systemic absorption and potential effects on the fetus. Human data on the safety of anticholinergic agents and on potential teratogenicity are lacking, but ipratropium bromide is not associated with teratogenicity in animal studies.69
Magnesium sulfate relaxes airway smooth muscle, most likely via its antagonism of calcium entry into airway smooth muscle cells.70 Its use is limited primarily to acute bronchospasm.71
Anti-Inflammatory Agents
Proposed mechanisms of action for corticosteroids are (1) decreases in cellular infiltration and mediator release, (2) reductions in airway permeability, and (3) up-regulation of the beta-adrenergic system.72 Unlike bronchodilators, corticosteroids not only reduce airway sensitivity to a constrictor stimulus73 but also decrease the maximal extent of airway narrowing, a feature that may predict severity of an acute asthmatic episode.74
The use of inhaled corticosteroids has gained popularity. This route of administration is effective and may limit fetal side effects. Studies have assessed the effects of systemic and inhaled corticosteroids on the fetus. Neither systemic nor inhaled corticosteroids have been proven to increase the risk for congenital malformations in humans. Inhaled corticosteroids do not affect glucocorticoid-regulated pathways in the fetus and therefore are unlikely to cause adverse effects on fetal growth and development.75 Although oral corticosteroid use is associated with an increased incidence of LBW infants,76 inhaled corticosteroids do not appear to increase perinatal risk.77 Further, a meta-analysis did not show an association between inhaled corticosteroid use and any adverse perinatal outcome.78 Of interest, use of inhaled corticosteroids during pregnancy can be guided by measurements of exhaled nitric oxide, which, in a randomized trial, were shown to significantly reduce exacerbations when compared with use of a clinical algorithm based on symptoms alone.79
Corticosteroids may increase perinatal morbidity by exacerbating maternal glucose intolerance, especially in women who also receive treatment with a beta-adrenergic agonist. Thus, careful monitoring of maternal glucose concentration is indicated in asthmatic women who require treatment with a corticosteroid during pregnancy. However, because of the efficacy of corticosteroids in controlling severe asthma during pregnancy, these agents should not be withheld from the medical regimen.
Some authorities have recommended that corticosteroid-dependent asthmatic women receive large doses of parenteral corticosteroids during labor to prevent complications related to adrenal suppression.55,80,81 The scientific basis for this recommendation is questionable. Although physiologic glucocorticoid replacement reduced hemodynamic instability and mortality in adrenalectomized primates that underwent surgery, supraphysiologic doses provided no additional benefit.82 Furthermore, inhaled corticosteroids in moderate doses do not produce adrenocortical suppression.83 There is little information about the benefit of corticosteroid replacement therapy during labor. The potential for adrenal insufficiency in infants of asthmatic mothers taking inhaled or oral corticosteroids appears to be very low,81 most likely owing to the widespread use of either prednisone or prednisolone. In the mother, prednisone is converted rapidly to prednisolone, which crosses the placental barrier to a very limited extent.
Cromolyn sodium and nedocromil sodium belong to a class of drugs that are thought to reduce inflammation and mediator release primarily by stabilizing mast cells and perhaps other inflammatory cells. Nedocromil also inhibits cellular chloride ion flux, a feature that may explain its ability to affect a range of airway cells, including nerve cells.84
Cromolyn and nedocromil are administered as aerosols. Limited studies suggest that cromolyn is safe during pregnancy,85 and clinical experience is greater with cromolyn than with nedocromil. Thus, use of cromolyn is preferred.
On the basis of the observation that leukotrienes are released into the airways by immune cells and contribute to the inflammatory process, other forms of anti-inflammatory therapy are leukotriene receptor antagonists and leukotriene synthesis inhibitors. Safety data for the use of these agents in pregnancy are scarce. Bracken et al.47 did not observe adverse neonatal outcomes in nine women exposed to these agents. A later prospective study of 96 women showed that use of leukotriene receptor antagonists was not associated with a specific pattern of congenital abnormalities, but the investigators cautioned that extrapolation of the data to a large population would require additional studies because of the limited sample size of the study.85
Obstetric Management
The following aspects of obstetric management of the asthmatic parturient may differ from that of the nonasthmatic patient: (1) induction of labor, (2) management of postpartum hemorrhage, and (3) treatment of hypertension.
For induction of labor, prostaglandins should be administered cautiously in women with asthma. Prostaglandin F2α constricts airways in vivo86 and in vitro.87 Airways of asthmatic subjects demonstrate greater sensitivity to prostaglandin F2α, and its use to induce labor is associated with bronchospasm.88 Prostaglandin E2 can have either dilating or constricting effects on the airways, perhaps because of its ability to activate a variety of different types of prostaglandin receptors.89 Because of the known risk for bronchospasm after exposure to prostaglandin F2α and the possible risk after exposure to prostaglandin E2, alternative methods of induction of labor are preferred in asthmatic women.
Likewise, asthma represents a relative contraindication to the administration of 15-methyl prostaglandin F2α (carboprost, Hemabate) for the treatment of postpartum hemorrhage. The use of ergot alkaloids to treat postpartum hemorrhage in asthmatic women has also been questioned. Although controlled studies have not been performed, ergot alkaloids have been associated with episodes of acute bronchospasm,90,91 on the basis of either their tryptaminergic actions or their ability to activate α1-adrenergic receptors on airway smooth muscle cells. Oxytocin, which does not significantly affect airway tone, is the preferred ecbolic agent in asthmatic patients.
Beta-adrenergic receptor antagonists are used to treat hypertension in some pregnant women. In asthmatic women, these agents may provoke bronchospasm when used acutely.92 Other antihypertensive agents, such as hydralazine and sodium nitroprusside, do not seem to enhance airway responsiveness.
Anesthetic Management
Preoperative Assessment
During the preoperative evaluation, the anesthesia provider should assess the severity of the disease and whether an acute asthmatic episode is present. The medical history should include information about symptoms of wheezing, dyspnea, and cough. Further information should be sought about the frequency and severity of symptoms, the course of these symptoms during pregnancy, and the date of the most recent exacerbation. Patients who have frequent, severe attacks are at increased risk for morbidity in the peripartum period.
Physical examination should focus on the pulmonary system. Chest auscultation may demonstrate wheezing with or without a prolonged expiratory phase. However, wheezing may not be audible if air movement is markedly reduced. Additional signs of an acute exacerbation of asthma include tachypnea, an exaggerated (> 20 mm Hg) pulsus paradoxus, and the use of accessory respiratory muscles.
In a pregnant woman with stable asthma, laboratory tests add little to anesthetic management. However, if an acute exacerbation is suspected, chest radiographic examination, arterial blood gas measurements, and pulmonary function tests may assist with diagnosis and therapy. Chest radiographic examination helps diagnose precipitating or complicating conditions such as pneumonia, pneumothorax, and heart failure. During an episode of acute asthma, arterial blood gas measurements
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


