Fig. 6.1
Populations of ACh vesicles in the terminal nerve space (RRP: rapid release pool, recycling pool, reserve pool)
Released ACh binds to receptors (AChRs) on the post-synaptic membrane (two molecules can bind to each receptor). AChRs are sodium channels, and ACh binding produces a local depolarization or change in conductance of the postsynaptic membrane. This local depolarization of the muscle membrane is referred to as an excitatory postsynaptic potential or an endplate potential (EPP). The depolarization of one AChR does not generate a sufficient EPP to generate a postsyaptic action potential. This is important since, even in the absence of synaptic transmission there is a baseline spontaneous release of a small number of vesicles (1–5/s) which leads to tiny postsynaptic depolarizations called miniature endplate potentials that are of no physiologic significance.
The change in membrane potential associated with each EPP will decrease with time and distance. Just like the ripples caused by a stone dropped into water the size of each ripple decreases as it spreads outwards until it eventually disappears. However adjacent EPPs are additive or summative when they occur simultaneously. The larger the number of ACh-bound receptors the greater the total EPP. Eventually a critical level of depolarization is reached, known as the threshold potential. When the threshold is surpassed at the endplate, an action potential is triggered in the muscle fiber.
To ensure that NM transmission occurs reliably, the endplate depolarization that is typically induced is usually much higher than this threshold. The excess of amplitude is called the safety factor. This amplitude depends on the quantity of synthesis and exocytosis of ACh and on the state of the ACh receptors. In newborns and premature infants, the safety factor of the endplate is reduced due to immaturity of the NMJ. They generate fewer endplate potentials, less quanta inside the vesicles, and prolonged durations of the individual endplate potentials and of the total endplate potential [29].
Repetitive Nerve Stimulation (RNS) Tests
Repetitive stimulation (RNS) of a nerve mimics certain types of exercise and modifies the safety factor of the NMJ. This phenomenon can be used to explore different types of abnormalities affecting the NMJ. In the setting of disorders of the neuromuscular junction, repetitive stimulation of a motor nerve may trigger a decrement or facilitation in the amplitudes of successive compound motor action potentials, the exact response depending in large part on the frequency of the stimulations [26, 30]. RNS may be performed at low frequencies (2–3 Hz) for a train of 6–10 supramaximal responses or at high frequencies (20–50 Hz) for a few seconds or minutes; 10 Hz is generally regarded as the boundary between low and high frequency, but to avoid ambiguity regarding the expected response, repetitive stimulation is rarely performed at 10 Hz. The results of RNS may be influenced by short (10 s) or long (1 or more minutes) exercise. It is difficult to elicit satisfactory voluntary sustained muscle contraction in infants and younger children. In children with suspected MG, RNS at rest is usually sufficient and any tolerance a child may have for additional testing should be used to examine other muscle-nerve combinations rather than post-exercise testing of a limited selection [31]. A short train of high frequency (20-50 Hz) repetitive stimulation for 1–2 s is generally regarded as mimicking brief maximal exercise and is typically followed by a standard train of low-frequency repetitive stimulation at 2–3 Hz; however, even a short duration of high frequency stimulation is quite uncomfortable, and in children conscious sedation or general anesthesia is generally regarded as being required for any high-frequency repetitive stimulation.
The choice of the sites examined is a key factor, and it is important to explore a broad range of anatomical sites, bilaterally when possible [32]. Examining those muscles that are clinically weak at the time of testing should be prioritized as that strategy will increase the yield of testing. The most accessible nerve-muscle pairs to study are in the limbs, including ulnar nerve recording abductor digiti minimi (ADM), the most commonly studied extremity site; median nerve to abductor pollicis brevis (APB); and peroneal nerve to extensor digitorum brevis (EDB) or tibialis anterior (TA). However, these sites may be less sensitive to abnormalities due to the topography of the symptoms or to the lower temperature of the limbs compared to more proximal muscles. To increase the yield, the neurophysiologist should ensure that the limbs have been adequately warmed prior to testing. Movement artifacts are also more likely to happen in the limbs and therefore a gentle stabilization of the extremity with reassurance and distraction are usually necessary to reduce the chances of the child introducing artifact by pulling away or flinching. Standardized stabilization bands for the stimulation electrode may be used to prevent slippage of the stimulator. Certain muscles sometimes studied in adults are too small to be examined in young children (e.g., anconeus) [28, 32]. Ulnar nerve studies recording ADM are less technically challenging and less prone to the introduction of artifact, particularly in a cooperative patient.
Examination of more proximal nerve-muscle pairs such as spinal accessory nerve recording trapezius and facial nerve recording nasalis, frontalis, or orbicularis oculi often will have a higher yield and should be included in the set of repetitive nerve stimulation studies whenever possible. Spinal accessory nerve stimulation to the trapezius is best performed by placing the recording (G1) electrode on the thicker bulk of the muscle (half way between the neck and the acromion process). This is a simple and useful test because the nerve is very accessible in the upper border of the sternocleidomastoid muscle in the neck and supramaximal stimulation can typically be reached at low stimulation intensities. A comfortable position may be obtained if the child is lying supine, with a slight elevation of the head on a pillow and the neck turned towards the contralateral side facing a parent, who may help distract and calm the child while stabilizing the head. Gentle stabilization of the shoulders and trunk may also be necessary. For facial nerve recordings, stimulation is performed in the pre-auricular area and the recording electrode may be placed on the nasal muscles (lateral to the nose), on the lateral orbicularis oculi muscle, or sometimes frontalis. Facial nerve studies may be difficult to interpret in children as compound motor amplitude potentials (CMAPs) tend to be quite small (sometimes well below 1 mV), which can present technical challenges when attempting to track CMAP amplitudes serially. Younger children may not tolerate repetitive stimulation at these sites as well as the extremity sites. However, since proximal muscles are often involved early and more severely in some disorders of neuromuscular transmission (e.g., juvenile myasthenia gravis), proximal sites are often important to check, especially if bulbar or facial symptoms are present [26].
Other recording sites may be of particular interest in certain settings, including the phrenic nerve in the ICU or in mechanically ventilated patients [33], and the hypoglossal nerve [34]. In addition to temperature and movement artifacts, other technical factors should be considered. Treatments that enhance the function of the neuromuscular junction, in particular acetylcholinesterase inhibitors, should be held for at least 6 h prior to RNS testing to maximize the potential diagnostic yield. It is important to remember that normal RNS does not always exclude a NMJ disorder, especially for specific subcategories such as ocular juvenile myasthenia gravis. Single fiber EMG is more sensitive for some disorders of neuromuscular transmission and will be discussed in Chap. 10.
Low Frequency (Slow) Repetitive Nerve Stimulation
Low frequency (also known as slow) RNS is performed at 2 or 3 Hz. The amplitudes of the negative phase of the first and fourth compound motor action potentials (CMAP) are measured from baseline to negative peak, and the percent change of the fourth response compared with the first is defined as the decrement or increment. A CMAP decrement of greater than 10% is accepted as abnormal, assuming that a smooth progression of the response amplitude train and reproducibility are present. Children may tolerate the studies better when the number of stimuli per train are limited. One popular protocol consists of trains of 6 stimuli at 2 Hz.
A normal recording without artifact will show little variation in CMAP amplitudes throughout the train. Low frequency RNS at 2–3 Hz will progressively deplete the primary store of ACh quanta, and fewer quanta will be released with each consecutive stimulation. The corresponding total EPP will fall in amplitude, but in a healthy individual the large safety factor ensures that even the reduced EPPs are larger than threshold, thus the resulting muscle fiber action potential is preserved throughout the recording. Within seconds, the secondary stores will be mobilized to replenish the primary ones, thus ensuring that the threshold potential is always surpassed. In pathologic conditions of the neuromuscular junction, the safety factor is reduced, so that normally tolerated drops in the EPP will fall below the required threshold potential, thus leading to failure of synaptic transmission and the absence of the expected muscle fiber action potential in some muscle fibers. This development gives rise to the decrement that is observed when looking at the overall CMAP amplitude generated by that muscle (Fig. 6.2). These concepts form the basis of the decrements with slow RNS that are seen in NMJ disorders.
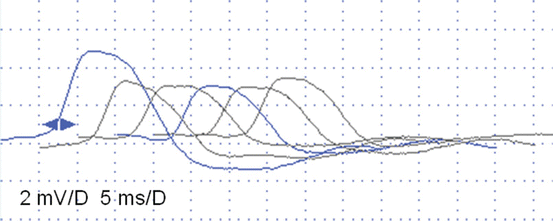

Fig. 6.2
RNS at 2 Hz in a 6 year–old girl with a congenital myasthenic syndrome (de novo mutation in CHRND). The child was mechanically ventilated via tracheostomy and fed by gastrostomy and suffered severe muscle weakness from the first months of life. RNS shows an abnormal decrement (30%) observed between the first and fourth stimulations in the phrenic nerve. The study was performed under sedation and the mechanical ventilation was stopped for a few seconds during the test to prevent ventilation artifact
High Frequency (Rapid) Repetitive Nerve Stimulation Tests
High frequency (also known as rapid) RNS (10–50 Hz) is particularly valuable for detecting disorders affecting the pre synaptic processes of neuromuscular transmission. After release of quanta from the presynaptic terminal, mobilization of additional quanta from the secondary store is the primary physiologic means of replenishing presynaptic acetylcholine stores. In the setting of high frequency repetitive stimulation, which is non-physiologic (i.e., does not occur in the setting of natural neuromuscular transmission), the mobilization of additional quanta is complemented by the accumulation of intracellular calcium. At frequencies over 10 Hz, the calcium pump is not able to pump calcium ions back into the extracellular synapse before the next stimulation, and thus abnormally high intracellular calcium concentrations occur, leading to increased release of quanta and a correspondingly higher EPP. In normal individuals, higher EPPs are non-consequential, as the same all-or-none muscle fiber action potentials are generated. In pathologic conditions where the baseline EPP is below threshold for some muscle fibers and a muscle fiber action potential is not generated for affected muscle fibers, the baseline CMAP amplitude is reduced. In those circumstances, the higher presynaptic intracellular calcium concentrations associated with rapid RNS (20–50 Hz) increases the number of muscle fibers that reach threshold, resulting in a higher CMAP amplitude and the phenomenon of facilitation associated with presynaptic disorders of neuromuscular transmission (Fig. 6.3).


Fig. 6.3
Slow RNS (3 Hz) (a) and Rapid RNS (30 Hz) (b) in a 3-year-old boy with severe congenital hypotonia, intellectual disability, epilepsy, and low-amplitude CMAPs on standard nerve conduction studies (setting 2 mV/5 ms). No significant decrement is observed at 3 Hz (a), but 30 Hz stimulation is associated with a striking increment (+154% from first to tenth CMAP) indicating a dysfunction of the NMJ, probably pre-synaptic. This patient’s clinical diagnosis was Lambert-Eaton like syndrome, with an unknown etiology
Disorders of Membrane Excitability (Muscle Channelopathies) and Exercise Tests
Familial periodic paralyses and non-dystrophic myotonias are disorders of skeletal muscle excitability caused by mutations in genes coding for voltage-gated ion channels (so called “channelopathies”). These channels involve various cations (sodium, potassium, calcium) or anion (chloride) channels which play an important role in depolarizing the muscle membrane. Muscle channelopathies are characterized by episodic failure of motor activity due to muscle weakness (paralysis) or stiffness (myotonia). Clinical studies have identified three distinct forms of myotonias: recessive and dominant forms of myotonia congenita (MC), paramyotonia congenita (PC), and potassium-aggravated myotonia (PAM); and two forms of periodic paralyses: hyperkalemic (hyperPP) and hypokalemic (hypoPP) periodic paralyses, based on changes in blood potassium levels during the attacks. Both the recessive and dominant forms of myotonia congenita arise from mutations in CLCN1, which encodes a chloride channel that is found in skeletal muscle. Paramyotonia congenita (PC) and potassium-aggravated myotonia (PAM) are associated with mutations in SCN4A. Two genes have been implicated in periodic paralysis, SCN4A and CACNA1S. There are several factors that can make diagnosis difficult. Some affected patients will display no symptoms aside from myotonia. Clinical signs could also be transient, and influenced by various environmental variables such as temperature, exercise, and potassium ingestion. Furthermore, multiple clinical phenotypes (PC + hyperPP for example) may be observed in the same patient [4, 35, 36].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


