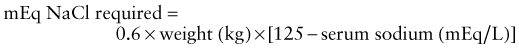Chapter 529 Renal Failure
529.1 Acute Renal Failure
Acute renal failure (ARF), also termed acute renal insufficiency, is a clinical syndrome in which a sudden deterioration in renal function results in the inability of the kidneys to maintain fluid and electrolyte homeostasis. ARF occurs in 2-3% of children admitted to pediatric tertiary care centers and in as many as 8% of infants in neonatal intensive care units. A classification system has been proposed to standardize the definition of acute kidney injury in adults. These criteria of risk, injury, failure, loss, and end-stage renal disease were given the acronym of RIFLE. A modified RIFLE criteria (pRIFLE) has been developed to characterize the pattern of acute kidney injury in critically ill children (Table 529-1). Because RIFLE focuses on glomerular filtration rate (GFR), a modification (Acute Kidney Injury Network, AKIN) categorizes severity by rise in serum creatinine: stage 1 >150%, stage II >200%, stage III >300%.
Table 529-1 PEDIATRIC-MODIFIED RIFLE (PRIFLE) CRITERIA
| CRITERIA | ESTIMATED CCl | URINE OUTPUT |
|---|---|---|
| Risk | eCCl decrease by 25% | <0.5 mL/kg/hr for 8 hr |
| Injury | eCCl decrease by 50% | <0.5 mL/kg/hr for 16 hr |
| Failure | eCCl decrease by 75% or eCCl <35 ml/min/1.73 m2 | <0.3 mL/kg/hr for 24 hr or anuric for 12 hr |
| Loss | Persistent failure >4 wk | |
| End-stage | End-stage renal disease (persistent failure >3 mo) |
eCCl, estimated creatinine clearance; pRIFLE, pediatric risk, injury, failure, loss and end-stage renal disease.
Pathogenesis
ARF has been conventionally classified into 3 categories: prerenal, intrinsic renal, and postrenal (Table 529-2).
Table 529-2 COMMON CAUSES OF ACUTE RENAL FAILURE
PRERENAL
INTRINSIC RENAL
POSTRENAL
Intrinsic renal ARF includes a variety of disorders characterized by renal parenchymal damage, including sustained hypoperfusion and ischemia. Many forms of glomerulonephritis, including postinfectious glomerulonephritis, lupus nephritis, Henoch-Schönlein purpura nephritis, membranoproliferative glomerulonephritis, and anti-glomerular basement membrane nephritis, can cause ARF. Hemolytic-uremic syndrome (HUS) has been described as the most common cause of intrinsic ARF in the USA (Chapter 512).
Tumor lysis syndrome is a specific form of ARF related to spontaneous or chemotherapy-induced cell lysis in patients with lymphoproliferative malignancies. This disorder is primarily caused by obstruction of the tubules by uric acid crystals (Chapters 489 and 490). Acute interstitial nephritis is an increasingly common cause of ARF and is usually a result of a hypersensitivity reaction to a therapeutic agent or various infectious agents (Chapter 526).
Clinical Manifestations and Diagnosis
The physical examination must be thorough, with careful attention to volume status. Tachycardia, dry mucous membranes, and poor peripheral perfusion suggest inadequate circulating volume and the possibility of prerenal ARF (Chapter 54). Peripheral edema, rales, and a cardiac gallop suggest volume overload and the possibility of intrinsic ARF from glomerulonephritis or ATN. The presence of a rash and arthritis might suggest systemic lupus erythematosus (SLE) or Henoch-Schönlein purpura nephritis. Palpable flank masses might suggest renal vein thrombosis, tumors, cystic disease, or urinary tract obstruction.
Laboratory Findings
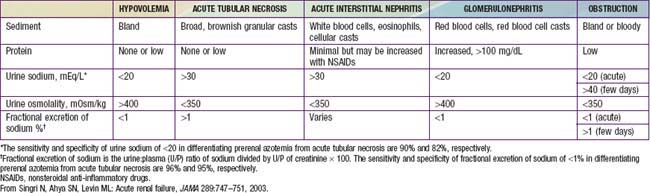
Urinary indices may be useful in differentiating prerenal ARF from intrinsic ARF (Table 529-3). Patients whose urine shows an elevated specific gravity (>1.020), elevated urine osmolality (UOsm > 500 mOsm/kg), low urine sodium (UNa < 20 mEq/L), and fractional excretion of sodium (FENa) <1% (<2.5% in neonates) most likely have prerenal ARF. Those with a specific gravity of <1.010, low urine osmolality (UOsm < 350 mOsm/kg), high urine sodium (UNa > 40 mEq/L), and FENa > 2% (>10% in neonates) most likely have intrinsic ARF.
Treatment
Medical Management
Determination of the volume status is of critical importance when initially evaluating a patient with ARF. If there is no evidence of volume overload or cardiac failure, intravascular volume should be expanded by intravenous administration of isotonic saline, 20 mL/kg over 30 min. In the absence of blood loss or hypoproteinemia, colloid-containing solutions are not required for volume expansion. Severe hypovolemia may require additional fluid boluses (Chapters 53, 54, and 64). Determination of the central venous pressure may be helpful if adequacy of the blood volume is difficult to determine. After volume resuscitation, hypovolemic patients generally void within 2 hr; failure to do so points to intrinsic or postrenal ARF. Hypotension due to sepsis requires vigorous fluid resuscitation followed by a continuous infusion of norepinephrine.
In ARF, rapid development of hyperkalemia (serum potassium level >6 mEq/L) can lead to cardiac arrhythmia, cardiac arrest, and death. The earliest electrocardiographic change seen in patients with developing hyperkalemia is the appearance of peaked T waves. This may be followed by widening of the QRS intervals, ST segment depression, ventricular arrhythmias, and cardiac arrest (Chapter 52.4). Procedures to deplete body potassium stores should be initiated when the serum potassium value rises to >6.0 mEq/L. Exogenous sources of potassium (dietary, intravenous fluids, total parenteral nutrition) should be eliminated. Sodium polystyrene sulfonate resin (Kayexalate), 1 g/kg, should be given orally or by retention enema. This resin exchanges sodium for potassium and can take several hours to take effect. A single dose of 1 g/kg can be expected to lower the serum potassium level by about 1 mEq/L. Resin therapy may be repeated every 2 hr, the frequency being limited primarily by the risk of sodium overload.
Mild metabolic acidosis is common in ARF because of retention of hydrogen ions, phosphate, and sulfate, but it rarely requires treatment. If acidosis is severe (arterial pH < 7.15; serum bicarbonate < 8 mEq/L) or contributes to hyperkalemia, treatment is required. The acidosis should be corrected partially by the intravenous route, generally giving enough bicarbonate to raise the arterial pH to 7.20 (which approximates a serum bicarbonate level of 12 mEq/L). The remainder of the correction may be accomplished by oral administration of sodium bicarbonate after normalization of the serum calcium and phosphorus levels. Correction of metabolic acidosis with intravenous bicarbonate can precipitate tetany in patients with renal failure as rapid correction of acidosis reduces the ionized calcium concentration (Chapter 52).
Hypertension can result from hyperreninemia associated with the primary disease process and/or expansion of the extracellular fluid volume and is most common in ARF patients with acute glomerulonephritis or HUS. Salt and water restriction is critical, and diuretic administration may be useful (Chapter 439). Isradipine (0.05-0.15 mg/kg/dose, maximum dose 5 mg qid) may be administered for relatively rapid reduction in blood pressure. Longer-acting agents such as calcium channel blockers (amlodipine, 0.1-0.6 mg/kg/24 hr qd or divided bid) or β-blockers (propranolol, 0.5-8 mg/kg/24 hr divided bid or tid; labetalol, 4-40 mg/kg/24 hr divided bid or tid) may be helpful in maintaining control of blood pressure. Children with severe symptomatic hypertension (hypertensive urgency or emergency) should be treated with continuous infusions of sodium nitroprusside (0.5-10 µg/kg/min), labetalol (0.25-3.0 mg/kg/hr), or esmolol (150-300 µg/kg/min) and converted to intermittently dosed antihypertensives when more stable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree