Chapter 52
Renal Disease
Yaakov Beilin MD
Chapter Outline
“Children of women with renal disease used to be born dangerously or not at all—not at all if their doctors had their way.”1 This statement describes early experiences with maternal renal disease and pregnancy outcome. It remains true that renal disease, either preexisting or occurring during gestation, may impair maternal and fetal health. Experience and investigations during the past three decades have significantly improved the outcome for pregnant women with renal disease.2
Physiologic Changes in Pregnancy
A review of the renal physiologic changes that occur during normal pregnancy is helpful to understand and evaluate coexisting renal disorders (see Chapter 2). Early in gestation, increased intravascular volume leads to renal enlargement. Hormonal changes result in dilation of the renal pelvis and ureters; dilation often is accompanied by decreased ureteral peristalsis. Dilated uterine and ovarian veins, and the gravid uterus, may obstruct ureter drainage at the pelvic brim. Together, these changes predispose pregnant women to vesicoureteric reflux and ascending infection. Alterations in glomerular hemodynamics and tubular function also occur. Increased cardiac output and decreased intrarenal vascular resistance cause an 80% increase in renal blood flow and a 50% increase in glomerular filtration rate (GFR) during pregnancy. These changes are somewhat less pronounced near term. Because of the increased GFR, a serum creatinine concentration greater than 0.6 to 0.8 mg/dL and a blood urea nitrogen (BUN) concentration greater than 8 to 9 mg/dL (upper limit of normal for the pregnant patient) suggest renal insufficiency in the pregnant woman. Tubular sodium reabsorption and osmoregulation are reset, allowing a “physiologic hypervolemia” during gestation. Modest proteinuria, up to 300 mg in 24 hours, also occurs during pregnancy.3
Urinary tract infections (see Chapter 37) and renal dysfunction associated with hypertensive disorders of pregnancy (see Chapter 36) are discussed elsewhere in this text.
Renal Parenchymal Disease
Definition and Pathophysiology
Renal parenchymal disease consists of two general groups of disorders, glomerulopathies and tubulointerstitial disease. Glomerulopathies are further subdivided into disorders that involve inflammatory or necrotizing lesions—the nephritic syndromes—and disorders that involve abnormal permeability to protein and other macromolecules—the nephrotic syndromes. More than 20 specific glomerulopathies exist. The nomenclature for these glomerulopathies is complex, and specific diseases are not discussed in detail here.
Tubulointerstitial diseases are disorders characterized by abnormal tubular function. They result in abnormal urine composition and concentration but are not characterized by decreased GFR until late in the disease course. The disorders in this category include interstitial nephritis, renal cystic disease, renal neoplasia, and functional tubular defects.
Patients with renal parenchymal disorders may remain asymptomatic for years, and they may exhibit only proteinuria and microscopic hematuria, with little if any evidence of reduced renal function. Spontaneous recovery or improvement with treatment occurs with many glomerulopathies. However, other patients exhibit progressive nephropathy, hypertension, and renal insufficiency. The incidence of kidney disease in pregnancy is approximately 0.12%.4 In two thirds of these patients, the disorder results from glomerulopathy, and in one third, from tubulointerstitial disease.5
Diagnosis
Women with preexisting disease may choose to become pregnant without the counsel of their nephrologist. When such patients become pregnant, the obstetrician and nephrologist seek to define the extent of renal involvement. Serial blood pressure measurements are obtained to define the severity of hypertension and the efficacy of current antihypertensive therapy. Creatinine clearance and the level of proteinuria should be determined. Urinalysis yields information about the presence of renal casts and bacteriuria. The determination of serum creatinine and BUN concentrations defines the extent of renal insufficiency. A serum creatinine concentration greater than 0.8 mg/dL, which may be normal in the nonpregnant woman, may represent significant renal insufficiency during pregnancy. Alternatively, the obstetrician may first detect renal dysfunction through routine prenatal screening tests. If proteinuria, hematuria, or azotemia is detected, a complete biochemical evaluation should be performed.
Both preeclampsia and renal disease may manifest as hypertension, proteinuria, and edema. The distinction between the two disorders is often unclear, especially after 20 weeks’ gestation. Fisher et al.6 evaluated 176 renal biopsy specimens obtained from hypertensive women immediately postpartum, most of whom had a clinical diagnosis of preeclampsia. The clinicopathologic correlation was poor. Histologic evidence of preeclampsia (e.g., glomerular endotheliosis without hypercellularity) was present in only 65% of these hypertensive women. Primary renal disease was present in 20%, and hypertensive nephrosclerosis occurred in 11%. Nulliparous women (84%) were more likely to have a correct diagnosis of preeclampsia than parous women (38%).
Renal tissue biopsy is often used to establish a diagnosis in nonpregnant patients. Chen et al.7 reported a series of 15 percutaneous renal biopsies performed in 15 pregnant women with onset of renal dysfunction of unknown cause during pregnancy. All patients underwent biopsy before 30 weeks’ gestation. A biopsy-related complication occurred in only one patient who experienced gross hematuria. The authors judged that histologic results provided useful clinical guidance that facilitated successful fetal outcome in 14 of the pregnancies. More recently, Day et al.8 performed renal biopsy in 20 pregnant women; the biopsy results led to altered management in 9 of 20. One patient had minor hematuria that resolved spontaneously. In contrast, Kuller et al.9 reviewed 18 renal biopsies performed during pregnancy (n = 15) or in the immediate postpartum period (n = 3) in 15 women with elevated blood pressure. After the biopsy, renal hematoma was identified in 7 (39%) women, and 2 (11%) women required blood transfusion. Four intrauterine fetal deaths occurred, although none was a direct result of the biopsy. It is possible that women with elevated blood pressure are at a greater risk for postbiopsy complications. Because renal biopsy exposes the pregnant woman to potential complications, many perinatologists recommend biopsy only when sudden deterioration in renal function or symptomatic nephrotic syndrome occurs before 28 weeks’ gestation, at which time definitive diagnosis may guide appropriate treatment. For problems beyond 28 weeks’ gestation, the recommendation is to delay biopsy until the postpartum period.8,9
Effect of Pregnancy on Preexisting Kidney Disease
The extent to which pregnancy affects preexisting renal disease depends on the level of renal insufficiency before pregnancy. Among women with mild antenatal renal insufficiency, pregnancy does not substantially alter the natural course of renal disease.10,11 Jungers et al.10 evaluated the effect of pregnancy on renal function among 360 women with primary glomerulonephritis. During the study period, 171 (48%) women became pregnant. All study subjects had normal renal function at the time of entry into this study, and all of the patients who became pregnant had normal renal function at conception. In this case-controlled study, pregnancy was not identified as a risk factor for progression to end-stage renal failure. Limardo et al.11 evaluated 223 women with biopsy-documented IgA nephropathy who had a serum creatinine level greater than 1.2 mg/dL, 136 of whom became pregnant. Women were observed for a minimum of 5 years (median, 10 years), and pregnancy did not seem to affect long-term outcome of kidney disease or the onset of proteinuria or hypertension.
In contrast, Jones and Hayslett12 analyzed the outcome of 82 pregnancies in 67 women with preexisting moderate or severe renal insufficiency (i.e., serum creatinine level > 1.4 mg/dL before pregnancy or at the first antepartum visit). The mean ± SD serum creatinine concentration increased from 1.9 ± 0.8 mg/dL in early pregnancy to 2.5 ± 1.3 mg/dL in the third trimester. The prevalence of hypertension rose from 28% at baseline to 48% during late pregnancy. Pregnancy-related deterioration of maternal renal function occurred in 43% of cases. Purdy et al.13 also found that greater than 40% of women with moderate to severe kidney disease had deterioration in renal function due to pregnancy. Women with a serum creatinine concentration greater than 2.0 mg/dL who became pregnant had a one-in-three chance of developing dialysis-dependent end-stage renal disease during or shortly after pregnancy.14
The pathophysiology by which pregnancy exacerbates renal disease is unknown. One hypothesis is that increased glomerular perfusion, which normally accompanies pregnancy, paradoxically causes further injury to the kidneys in patients with preexisting impairment of function. However, this hypothesis is unsupported by published data, which demonstrate no evidence of hyperfiltration (i.e., an initial decline in serum creatinine concentration) during early pregnancy in patients with renal disease.15 An alternative hypothesis is that preexisting renal disease may induce a cascade of platelet aggregation, microvascular fibrin thrombus formation, and endothelial dysfunction that leads to microvascular injury in the already tenuous kidneys.14
Effect on the Mother and Fetus
Pregnant women with chronic kidney disease are at an increased risk for maternal and fetal complications. Nevis et al.16 systematically reviewed all published observational studies of women with chronic kidney disease that included a control group for comparison, excluding retrospective studies. They identified 13 studies that included at least five women between 1966 and 2010. Maternal complications included gestational hypertension, preeclampsia/eclampsia, and maternal mortality. Adverse fetal outcomes included preterm births, fetal growth restriction (also known as intrauterine growth restriction), small-for-gestational-age infants, neonatal mortality, stillbirths, and low birth weight. Adverse maternal outcomes were found in 12 studies, and the incidence was five times greater than in women without kidney disease. Adverse fetal outcomes were identified in nine studies, and the incidence was two times greater than in the normal healthy women.
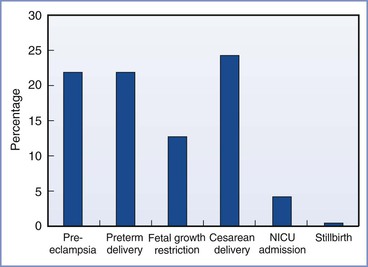
The incidence of obstetric complications is proportional to the extent of preexisting maternal renal disease and preexisting hypertension.17 Bar et al.18 evaluated maternal and neonatal outcomes in 38 women (46 pregnancies) with primary renal disease. They observed an increase in complications compared with healthy women (Figure 52-1). In a logistic regression model, only preexisting hypertension and a high preconception serum uric acid level predicted worse outcome. Other factors (e.g., degree of preexisting renal impairment) were not significant predictors, but, of note, 90% of the cohort had mild disease. In a study of women with moderate to severe renal disease, Jones and Hayslett found the complication rate was much greater.12 The incidence of preterm birth was 59%, the incidence of fetal growth restriction was 37%, and the cesarean delivery rate was 59%. Ramin et al.19 reviewed the literature for studies of renal disease and maternal and fetal outcome. Overall, fetal survival ranged from 64% to 98% depending on the extent of renal insufficiency and the presence or absence of hypertension.
Medical and Obstetric Management
During pregnancy, the nephrologist and the obstetrician monitor maternal renal function, blood pressure, and fetal development at frequent intervals. Monthly determination of serum creatinine concentration, creatinine clearance, and proteinuria allows the recognition of renal deterioration. An antepartum consultation with the anesthesiologist should also be considered.
Some glomerulopathies respond to corticosteroids, and corticosteroid therapy should be continued during pregnancy. Rapid deterioration of renal function that occurs before 28 weeks’ gestation may require renal biopsy to exclude rapidly progressive glomerulopathies that require treatment. Antihypertensive therapy should be instituted as needed (see Chapter 36). Recombinant human erythropoietin improves maternal anemia during pregnancy.20 Protein restriction places the fetus at risk for growth restriction and is not used. Deterioration of maternal renal function, the onset of preeclampsia, or evidence of fetal compromise may necessitate preterm delivery.
Hemodialysis and Long-Term Ambulatory Peritoneal Dialysis
When renal disease has progressed to end-stage renal failure (i.e., GFR < 5 mL/min), fertility is suppressed and conception and pregnancy are rare. Less than 10% of premenopausal patients undergoing dialysis have regular menses. Luteinizing hormone and follicle-stimulating hormone concentrations assume an anovulatory pattern, which causes 40% of affected women to be amenorrheic. Half of all female patients undergoing dialysis exhibit hyperprolactinemia because of reduced clearance and hypothalamic disturbances.21 Toma et al.22 surveyed 2504 dialysis units in Japan and reported only 172 pregnancies among 38,889 women who were undergoing dialysis.
There are two modalities of dialysis: extracorporeal hemodialysis and intracorporeal peritoneal dialysis. Hemodialysis necessitates vascular access and the need for anticoagulation of the extracorporeal circuit and may be complicated by cardiovascular instability, large fluid and electrolyte shifts, and the risk for hepatitis. Hypotension may compromise uteroplacental perfusion and cause fetal compromise. Even when hypotension and major fluid shifts are avoided, Doppler ultrasonographic examination of uterine and umbilical artery flow during hemodialysis suggests the occurrence of a redistribution of arterial flow away from the uteroplacental vascular bed.23 Fetal heart rate monitoring is recommended during dialysis.24 Rapid removal of maternal solutes and reduced oncotic pressure with attendant free-water diffusion into the amniotic cavity may lead to polyhydramnios. Hemodynamic consequences are minimized by more frequent but shorter dialysis runs. Long-term ambulatory peritoneal dialysis allows less hemodynamic trespass, a more stable fetal environment, and the freedom to undergo dialysis at home. However, peritoneal dialysis may not be associated with greater fetal survival. Complications of this modality include peritonitis and catheter difficulties.25
Published reports have noted a wide range of successful pregnancies in dialysis-dependent pregnant women, regardless of the modality of dialysis. Toma et al.22 reported that 90 (52%) of 172 pregnancies in women undergoing long-term hemodialysis were successful. More recently, because of improvement in maternal-fetal care, the success rate appears to be improving. Chou et al.26 pooled data from 10 published case series and 12 case reports and found that 71% of women undergoing hemodialysis and 64% of women undergoing peritoneal dialysis had a successful delivery. Similarly, Piccoli et al.27 pooled data from 10 studies that included 90 conceptions in 78 women and reported successful delivery can be achieved 75% of the time.
Maternal complications include malnutrition, anemia, and hypertension. Fetal complications include fetal growth restriction, fetal death, and preterm labor. BUN levels should be kept below 50 mg/dL before dialysis and below 30 mg/dL after dialysis.25 At birth, azotemia in the newborn is similar to that in the mother, but this quickly corrects because the newborn has normal kidney function. The long-term effects of intrauterine azotemia on newborn cognitive development are unknown.28
Patients undergoing hemodialysis have a high prevalence of viral hepatitis, a greater frequency of active tuberculosis, and a higher rate of infection with vancomycin-resistant enterococci, human immunodeficiency virus (HIV), and methicillin-resistant Staphylococcus aureus. The risk for hepatitis C virus (HCV) infection is particularly of concern, with reported rates as high as 36%. However, with improvement in aseptic technique and more attention to hand washing, the decline in the reuse of dialysis equipment, and the use of dedicated isolated dialysis machines for HCV-seropositive patients, the rates of infection and seroconversion can be markedly reduced.29,30
Anesthetic Management
Anesthetic management is influenced by the extent of renal dysfunction and hypertension. The parturient with stable renal disease, mild to moderate renal insufficiency, well-controlled hypertension, and euvolemia requires minimal special consideration. In contrast, the dialysis patient with end-stage renal failure presents many anesthetic challenges because renal disease may affect almost every organ system (Box 52-1). Poorly controlled hypertension leads to left ventricular hypertrophy and dysfunction. Symptoms of cardiovascular compromise should prompt echocardiography to evaluate ventricular function. An intra-arterial catheter also may aid the management of the parturient with poorly controlled hypertension. Uremic pericarditis, cardiomyopathy, and accelerated atherosclerosis are rarely seen until advanced uremia has been present for several years.
Normochromic, normocytic anemia secondary to impaired erythropoietin production, chronic gastrointestinal bleeding, and vitamin deficiency are common findings. Typically, the anemia is well tolerated and does not require transfusion, unless excessive surgical bleeding occurs. Uremic toxins may cause functional platelet defects; these abnormalities are reversed by dialysis. Thrombocytopenia may also occur as a result of increased peripheral destruction of platelets. Generalized coagulopathy may result from the anticoagulation used during the dialysis process.31 A full coagulation profile and careful bleeding history should be performed, especially before the initiation of neuraxial anesthesia. Hemodialysis fistulas should be padded carefully to prevent thrombosis. Blood pressure cuffs should not be placed on these extremities.
Neuraxial Anesthesia
Neuraxial anesthesia is the preferred technique for both labor analgesia and cesarean delivery, but there are some unique considerations in the parturient with renal disease. Uremic patients may be hypervolemic or hypovolemic, depending on the time elapsed since their last dialysis session. Hypovolemia and autonomic neuropathy may lead to profound hypotension during the initiation of sympathetic blockade. Intravascular volume should be assessed before induction of anesthesia. Assessment of clinical signs (e.g., skin turgor, mucous membranes, tachycardia) is generally sufficient. Central venous pressure monitoring or transthoracic echocardiography may be useful when the volume status remains unclear. Although there are no studies in the renal transplant patient, a role for intravenous prehydration to prevent hypotension is unlikely because this modality is not efficacious in the healthy parturient.32 There is insufficient evidence to recommend spinal versus epidural techniques for the patient with renal disease. Frequent monitoring of blood pressure and immediate treatment of hypotension is suggested.33 Preexisting peripheral neuropathy should be documented before the administration of neuraxial anesthesia.
Local anesthetic systemic toxicity (LAST) after bupivacaine brachial plexus blockade has been reported in patients with chronic renal failure.34 Whether LAST is related to toxic levels of local anesthetic unique to the renal failure patient is unclear. Rice et al.35 found no significant difference in the pharmacokinetic profile of bupivacaine after brachial plexus blockade in a group of uremic patients and in patients with normal renal function. There are no published data on the pharmacokinetics of epidurally administered local anesthetic agents in patients with chronic renal failure.
Orko et al.36 administered spinal anesthesia with plain bupivacaine 22.5 mg to 20 nonpregnant patients with chronic renal failure and 20 control patients. Maximal segmental anesthesia occurred more rapidly in the patients with renal disease (21 versus 35 minutes), but the duration was reduced. Further, the extent of sensory blockade was two segments higher in the patients with renal disease. There were no untoward effects in any of the patients.
General Anesthesia
Patients with chronic uremia exhibit delayed gastric emptying and hyperacidity, which may increase the risk for aspiration pneumonitis. In addition to sodium citrate, when time allows, the anesthesia provider also should consider administering a histamine-2 (H2)-receptor antagonist and metoclopramide. Recommended single doses for patients with renal failure are ranitidine 50 mg and metoclopramide 10 mg given intravenously. Weir and Chung37 suggested that patients with chronic renal failure present greater difficulties with tracheal intubation than otherwise healthy patients; however, an objective analysis of airway difficulty has not been performed in this population.
All the standard induction agents are safe in patients with renal failure. Etomidate may have an advantage because it supports the circulation better than other induction agents. Propofol exhibits normal volume of distribution and elimination in patients with renal failure and is also commonly used. Protein binding of propofol is unaffected by renal failure.38 Uremia increases blood-brain barrier permeability to many drugs.39 These changes may warrant a small reduction in the dose of propofol or thiopental for induction. The serum potassium concentration should be determined before induction of anesthesia. If the potassium concentration is greater than 5.5 mEq/L, dialysis should be performed before an elective procedure. Succinylcholine will cause a 0.5 to 0.7 mEq/L increase in potassium concentration, which is similar to the increment that occurs in patients without renal disease.40 If the patient is already hyperkalemic, this mild elevation may be sufficient to precipitate cardiac dysrhythmias. Plasma cholinesterase concentrations are normal, even after dialysis, and the duration of action of succinylcholine is not prolonged.41
Neuromuscular blockade should be maintained with an agent that does not rely on renal elimination. Cisatracurium undergoes Hofmann degradation, and therefore the duration of action is not prolonged in patients with renal failure. Hypermagnesemia, commonly found in patients with kidney disease, may potentiate neuromuscular blockade.42 Although anticholinesterase agents undergo renal elimination and have a prolonged duration in patients with renal insufficiency, the volume of distribution remains the same and standard doses are used for the reversal of neuromuscular blockade.
Postoperative Analgesia
The principles of postoperative analgesia for the woman with renal disease are the same as for healthy woman, with some important considerations because drug clearance can be altered for opioids and their metabolites (see Chapters 27 and 28). Morphine is generally safe as a single dose, but with longer-term use its metabolite, morphine-6-glucuronide, may accumulate. Meperidine is of particular concern because its active metabolite, normeperidine, is neurotoxic and is renally excreted. Hydromorphone and oxycodone, and their metabolites hydromorphone-3-glucuronide and α- and β-oxycodol, respectively, are also renally excreted and may accumulate with prolonged use. Methadone does not accumulate in patients with renal disease and may be a useful long-term analgesic. Fentanyl and sufentanil are only minimally excreted in the urine, and because they are short-acting drugs they may be particularly useful. Remifentanil is metabolized by blood and tissue esterases and is not dependent on the kidney for excretion, making it safe as well for use in patients with renal failure.43
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree