Background
Rates of HIV infections are increasing in older adults. Although it is known that the HIV/AIDS epidemics affects women disproportionately, little is known regarding immune functions in the genital tract of postmenopausal women, as relevant to HIV susceptibility.
Objective
The objective of the study was to compare levels of female reproductive tract immune mediators that are important for HIV-associated immune responses as well as intrinsic anti-HIV activity in the cervical vaginal lavages collected from HIV-negative pre- and postmenopausal women.
Study Design
Cervical vaginal lavage from 20 premenopausal and 20 postmenopausal women were assayed for interleukin-6, interleukin-8, tumor necrosis factor-α, secretory leukocyte protease inhibitor, elafin, human β-defensin-2, and macrophage inflammatory protein-3α using standard enzyme-linked immunosorbent assays. Anti-HIV activity of cervical-vaginal lavage was measured using TZM-bl indicator cells against HIV-1 IIIB and BaL. Whereas each postmenopausal woman provided only 1 sample, each premenopausal woman provided 3 samples, during proliferative, ovulatory, and secretory stages, based on menstrual dates.
Results
We observed significantly lower levels of tumor necrosis factor-α, MIP-3α, secretory leukocyte protease inhibitor, elafin, and human β-defensin-2 in cervical vaginal lavage from postmenopausal women compared with premenopausal women. Inhibition of HIV-1 infection was observed for both pre- and postmenopausal women, but cervical vaginal lavage from postmenopausal women showed significantly higher inhibition against HIV-1 BaL after adjusting for total protein concentration, genital pH, and reproductive tract infections. No change in mediators or HIV inhibition was observed through the stages of menstrual cycle. In addition, we observed that postmenopausal women with reproductive tract infections had significantly higher levels of tumor necrosis factor-α and significantly lower levels of interleukin-8, which were not observed in premenopausal women.
Conclusion
Our findings suggest that female reproductive tract immune microenvironment is distinct in HIV-negative postmenopausal women. Further studies are needed to assess the risk of HIV acquisition/transmission in this population.
Rates of new HIV diagnoses in the >50 year age group are on the rise and are characterized by poorer prognosis and shorter survival times. This may be due to unmeasured and overlooked risk behaviors in older adults that mimic those of younger age groups. Studies have shown that many older adults remain sexually active well into their 80s but often do not use condoms or get tested for sexually transmitted infections.
In the HIV-positive population in developed nations, an increasing trend is the growing number of people >50 years of age, primarily because of improved life expectancy resulting from the success of highly active antiretroviral therapy.
The HIV/AIDS epidemic affects women disproportionately. Because HIV/AIDS is often perceived as a condition having an impact on reproductive-age women, most epidemiological studies do not focus on older women. Therefore, little is known regarding the immunological mechanisms of HIV acquisition and transmission in postmenopausal women.
Immunologically, aging is characterized by a gradual immune senescence that includes inflamm-aging, a systemic increase in the levels of proinflammatory cytokines and decreased frequency and functionality of immune cells (reviewed elsewhere ). Aging affects the immune system in both sexes in a distinct manner; faster progression to immunosenescence has been demonstrated in men, whereas higher systemic inflammation and lower T cell function have been described in women (reviewed elsewhere ).
Sex-specific infections have been reported both in terms of incidence and disease severity and mortality. Whereas men show higher incidence rates against vector-borne diseases such as hantavirus, severity of disease is higher in women. Although mechanisms are ill defined, one reason for this may be the immune suppressive effects of testosterone in men vs the immune-enhancing effect of estrogen in women (reviewed elsewhere ).
Studies have shown chronic systemic inflammation in postmenopausal women, with higher levels of proinflammatory cytokines and a reduced ability to respond to pathogenic stimuli. Less is known regarding immune functions in the genital tract, which is critical to consider to prevent sexual acquisition/transmission of HIV. Furthermore, other factors that can affect the immune milieu of female reproductive tract including protein content, presence of infections, and vaginal pH also need to be considered in determining HIV susceptibility in women.
Given that the population of HIV-positive postmenopausal women and HIV-negative at-risk postmenopausal women is on the rise, understanding the immune responses in the female reproductive tract is of critical importance so that specific prevention/intervention strategies can be designed for this emerging high-risk population.
Materials and Methods
Ethical statement
Studies were conducted according to the principles expressed in the Declaration of Helsinki. This study was approved by the Miriam Hospital Institutional Review Board (Brown University, Providence, RI) on March 20, 2012 (protocol number 203312) as well as the George Washington University Institutional Review Board (Washington, DC) on March 22, 2012 (protocol number 031226). All patients provided written informed consent for the collection of samples and subsequent analysis.
Subject recruitment and study participants
All participants were recruited at The Miriam Hospital Research Center. Twenty premenopausal women who had regular menses for the last 3 months comprised the premenopausal group and 20 women who did not have their menses for at least 1 year were recruited for the postmenopausal group. One of the 20 postmenopausal women was subsequently excluded because it was suspected she was amenorrheic because of prolonged substance abuse.
The inclusion criteria were HIV-negative healthy women with a cervix, >18 years of age, no abnormal vaginal discharge or reproductive tract infection diagnosis in the last 2 weeks, no chronic medical conditions, immunizations, and not being on any immunomodulatory medications or antibiotics in past 2 weeks. At the screening visit, women were also negative for HIV, gonorrhea, chlamydia, and genital ulcers.
Women were excluded for if they were pregnant, if they were breast-feeding, if they had douched, if they used any vaginal products, or if they had sexual intercourse 48 hours prior to cervical-vaginal lavage collection. None of the premenopausal women were on hormonal contraceptives, and none of the postmenopausal women were on hormone replacement therapy.
Cervical-vaginal lavage collection and processing
Cervical-vaginal lavage was collected by rinsing the cervical-vaginal area with 10 mL of normal saline (BD Biosciences, Franklin Lakes, NJ) and processed immediately. Cervical-vaginal lavage was spun at 1500 × g for 10 minutes, supernatant removed, and respun at 2200 × g for 10 minutes. Supernatants were aliquoted, frozen at –80°C and batch shipped on dry ice to George Washington University for analyses.
Differential cell count was performed by making a cytocentrifuged slide of the cervical-vaginal lavage stained with Wright-Giemsa stain and read as a percentage of cell types of 100 cells counted.
To diagnose reproductive tract infections, we performed tests for HIV (OraQuick ADVANCE Rapid HIV-1/2 antibody test), gonorrhea, and chlamydia (urine polymerase chain reaction). Bacterial vaginosis was diagnosed as per current clinical parameters using Amsel’s criteria. A sample was considered bacterial vaginosis positive if it met at least 3 of the 4 criteria of >4.5 vaginal pH, presence of clue cells, milky discharge, and release of fishy odor after addition of 10% potassium hydroxide (whiff test).
We used an in-pouch culture for diagnosing Trichomonas vaginalis , which has clinical specificity of 100% and clinical sensitivity of 81-94% (Biomed Diagnostics, White City, OR).
Of the total 80 visits (60 premenopausal and 20 postmenopausal), there were 3 premenopausal Trichomonas vaginalis -positive visits, 2 of which were also bacterial vaginosis positive. Because of the small number of Trichomonas vaginalis -positive cases and the overlap of bacterial vaginosis and Trichomonas vaginalis within our small cohort, bacterial vaginosis and Trichomonas vaginalis were combined as a single reproductive tract infections group for analyses.
HIV viral stocks
HIV strains IIIB (CXCR4 tropic) and BaL (CCR5 tropic) were obtained from Dr P. Gupta (University of Pittsburgh, Pittsburgh, PA). Virus stocks were propagated in phytohemaglutinin-stimulated human peripheral blood mononuclear cells and stored frozen at −80° C. Virus titers were determined on TZM-bl cells.
Measurement of cytokines, chemokines, and antimicrobials in cervical-vaginal lavage
Cervical-vaginal lavage supernatants were stored at –80° C until assayed for secretory leukocyte protease inhibitor, tumor necrosis factor-α, interleukin-6, interleukin-8, macrophage inflammatory protein-3α (MIP-3α), and elafin using enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN). Human β-defensin-2 was assayed using an enzyme-linked immunosorbent assay kit from PeproTech (Rocky Hill, NJ). Quantification was based on standard curves obtained using a Microplate Reader (Biotek, Winooski, VT).
Determination of total protein concentration in cervical-vaginal lavage
Total protein concentration in each cervical-vaginal lavage sample was determined using the Pierce bicinchoninic assay protein assay kit (Thermo-Fisher Scientific), according to the manufacturer’s instructions.
Measurement of anti-HIV activity in cervical-vaginal lavage
Anti-HIV activity in cervical-vaginal lavage was determined using the TZM-bl indicator cell line (American Type Culture Collection, Manassas, VA). Cells were seeded at 2 × 10 4 cells/well in a 96-well plate and allowed to adhere overnight at 37°C. Cervical-vaginal lavage was diluted 1:4 in TZM-bl media (phenol red-free DMEM [Invitrogen Life Technologies, Carlsbad, CA]) supplemented with 10% defined fetal bovine serum (HyClone, Logan, UT), 2 mM L-glutamine (Invitrogen Life Technologies), and 50 μg/mL Primocin (Invivogen, San Diego, CA) and incubated with IIIB and BaL at 250 tissue culture infectious dose. The dose was selected to minimize virus-induced cytopathic effects while maintaining an ability to measure >1 log reduction in virus infectivity (standardized by that reported elsewhere ).
Samples were incubated with virus for 1 hour at 37°C and added to TZM-bl cells. Luciferase activity was measured for 48 hours upon application of substrate beta-Glo (Promega, Madison, WI). Uninfected cells and cells treated with cervical-vaginal lavage alone were used to determine background luminescence expressed as relative light units.
All conditions were tested in triplicates and repeated twice. To calculate percentage inhibition, relative light unit values of virus-only wells were averaged and set to 100%. Viability of cells upon treatment with cervical-vaginal lavage was quantified using the CellTiter 96 AQueous One solution cell proliferation assay (Promega) according to the manufacturer’s instructions. Briefly, reagent was added directly to the cell cultures and incubated for 30 minutes at 37°C followed by reading the plate in a plate reader at an optical density of 490 nm.
Statistical analyses
Markers in various groups were displayed using box plots. Values below the lower limit of detection were assigned the value of 1, and all values were log transformed. Because premenopausal women have repeated mediator measures collected up to 3 visits, all comparisons were based on generalized estimating equations. All models used an exchangeable working correlation structure. Because mediators generally have a skewed distribution, the generalized estimating equation models were based on logs ( Tables 1 and 2 ) or ranks (all other comparisons) of the mediators.
| Pre | Post | P value | |
|---|---|---|---|
| N | 20 | 19 | |
| General characteristics at the first visit | |||
| Age (all) | 35.1 ± 8.3 | 54.5 ± 8 | < .001 |
| Race | .771 | ||
| White | 10 (50%) | 9 (47.4%) | |
| Black | 5 (25%) | 4 (21.1%) | |
| Hispanic white | 2 (10%) | 4 (21.1%) | |
| Hispanic black | 1 (5%) | 0 (0%) | |
| Other/mixed | 2 (10%) | 2 (10.5%) | |
| Infections at any time | |||
| Trichomonas | 3 (15%) | 0 (0%) | .248 |
| BV | 9 (45%) | 8 (42.1%) | 1.000 |
| RTI | 10 (50%) | 8 (42.1%) | .863 |
| Clinical variables at the first visit | |||
| pH | 4.5 ± 0.4 | 4.6 ± 0.4 | .395 |
| WBC | 337 ± 496.1 | 202.1 ± 387.4 | .352 |
| Neutrophils | 0.93 ± 0.14 | 0.86 ± 0.33 | .363 |
| Mono/macrophages | 0.15 ± 0.18 | 0.08 ± 0.06 | .416 |
| Mediator | Premenopausal (n = 60) Median (IQR) | Postmenopausal (n = 19) Median (IQR) | P value | Cov-Ad P value | TP-Ad P value | Cov plus TP-Ad P value |
|---|---|---|---|---|---|---|
| Log 10 IL-6, pg/mL | 1.19 (0.46–1.38) | 1.00 (0.83–1.13) | .072 | .047 a | .125 | .100 |
| Log 10 IL-8, pg/mL | 2.35 (1.93–2.83) | 2.30 (1.97–2.74) | .754 | .590 | .739 | .907 |
| Log 10 TNF-α, pg/mL | 1.71 (1.47–1.86) | 0.99 (0.00–1.62) | < .001 a | < .001 a | < .001 a | < .001 a |
| Log 10 MIP-3α, pg/mL | 1.97 (1.77–2.38) | 0.00 (0.00–1.65) | < .001 a | < .001 a | < .001 a | < .001 a |
| Log 10 elafin, pg/mL | 5.58 (5.14–5.76) | 5.45 (5.21–5.65) | .512 | .575 | .855 | .942 |
| Log 10 SLPI, pg/mL | 5.38 (5.07–5.92) | 4.59 (4.23–4.77) | < .001 a | < .001 a | < .001 a | < .001 a |
| Log 10 HBD2, pg/mL | 3.83 (3.20–4.25) | 2.91 (1.87–3.88) | .018 a | .018 a | .047 a | .072 |
| Log 10 total protein, μg/mL | 2.28 (2.11–2.48) | 2.25 (1.92–2.32) | .114 | .154 | N/A | N/A |
| IIIB inhibition, % | 53.21 (38.13–73.24) | 45.49 (28.24–89.84) | .864 | .679 | .715 | .883 |
| BaL inhibition, % | 24.24 (1.52–32.58) | 28.93 (20.64–37.65) | .088 a | .007 a | .113 | .009 a |
Correlation analyses used Spearman rank correlations, and the correlation matrices were displayed as heat maps using pairwise-complete observations in which there were missing values. The correlations of pre- vs postmenopausal women were compared using a permutation test based on the Multiple Monte Carlo Test (MMCTest) procedure of Gandy and Hahn and using the method of Altman and Bland to account for repeated measures.
Results
Cohort characteristics and demographics
Cervical-vaginal lavage samples from 20 premenopausal and 19 postmenopausal women were analyzed. Each of the premenopausal women provided 3 samples coinciding with proliferative (days 5–9 following menses), ovulatory (days 12–16 following menses), and secretory (days 19–23 following menses) stages of their menstrual cycle. Each postmenopausal woman provided 1 sample.
The age range in premenopausal women was 23–48 years, (mean, 35 years). In postmenopausal women, the age range was 45–81 years, (mean, 55 years). For both groups, approximately 50% self-identified as white and 25% as black ( Table 1 ). Both groups had similar percentages of bacterial vaginosis (9 visits for premenopausal vs 8 visits for postmenopausal). However, 3 visits of Trichomonas vaginalis were all in the premenopausal group, with 2 of those with both bacterial vaginosis and Trichomonas vaginalis . Vaginal pH and presence of white blood cell count, neutrophils, and monocytes/macrophages were not significantly different ( Table 1 ).
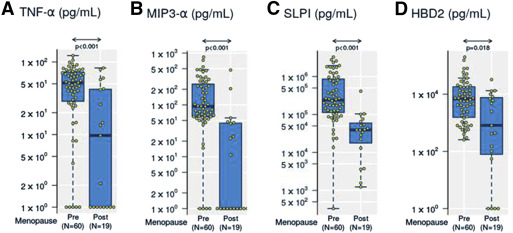
Reduced levels of soluble immune mediators in cervical vaginal lavage of postmenopausal women
To determine whether levels of immune mediators were altered in the genital tract secretions of postmenopausal women, we analyzed cervical-vaginal lavage samples for proinflammatory mediators interleukin-6, interleukin-8, and tumor necrosis factor-α, and anti-HIV/protective mediators MIP-3α, secretory leukocyte proteinase inhibitor, elafin, and human β-defensin-2.
We found significantly lower levels of tumor necrosis factor-α (median, 0.99 vs 1.7 log 10 pg/mL, P < .001) ( Figure 1 A and Table 2 ), MIP-3α (median 0 vs 1.97 log 10 pg/mL, P < .001) ( Figure 1 B and Table 2 ), secretory leukocyte proteinase inhibitor (median, 4.6 vs 5.4 log 10 pg/mL, P < .001) ( Figure 1 C and Table 2 ), and human β-defensin-2 (median, 2.9 vs 3.8 log pg/mL, P = .018) ( Figure 1 D and Table 2 ) in cervical-vaginal lavage from postmenopausal women compared with premenopausal women. All 4 mediators remained significantly reduced in postmenopausal women after normalization to the total protein concentration in cervical-vaginal lavage, genital pH and reproductive tract infections, bacterial vaginosis, and Trichomonas vaginalis ( Table 2 ). No significant changes were observed for other immune mediators measured ( Table 2 ).
When premenopausal samples were analyzed by cycle, we observed significantly higher levels of elafin in the secretory stage (median, 5.69 log 10 pg/mL), compared with the proliferative (median, 5.55 log 10 pg/mL) and ovulatory (median, 5.55 log 10 pg/mL) stages ( P = .028; Table 3 ). Similarly, levels of human β-defensin-2 were also significantly higher in the secretory stage (median, 3.86 log 10 pg/mL), relative to the proliferative (median, 3.81 log 10 pg/mL) and ovulatory (median 3.70 log 10 pg/mL) stages after adjustment for total protein concentration and after adjustment for total protein, genital pH, and reproductive tract infections ( P = .027 and P = .030, respectively; Table 3 ).
| Mediator | Proliferative (n = 20) Median (IQR) | Ovulatory (n = 20) Median (IQR) | Secretory (n = 20) Median (IQR) | P value | Cov-Ad P value | TP-Ad P value | Cov+TP-Ad P value |
|---|---|---|---|---|---|---|---|
| Log 10 IL, pg/mL | 1.23 (0.60–1.53) | 1.26 (0.71–1.43) | 1.14 (0.17–1.25) | .094 | .070 | .094 | .077 |
| Log 10 IL, pg/mL | 2.32 (1.91–3.22) | 2.45 (2.07–2.95) | 2.42 (1.98–2.71) | .340 | .365 | .720 | .662 |
| Log 10 TNF-α, pg/mL | 1.64 (1.39–1.89) | 1.7 (1.47–1.84) | 1.71 (1.62–1.85) | .513 | .393 | .623 | .491 |
| Log 10 MIP-3α, pg/mL | 2.05 (1.78–2.38) | 1.94 (1.78–2.36) | 1.97 (1.81–2.45) | .313 | .616 | .621 | .847 |
| Log 10 elafin, pg/mL | 5.55 (5.19–5.70) | 5.55 (5.15–5.73) | 5.69 (5.17–5.81) | .028 a | .024 a | .007 a | .006 a |
| Log 10 SLPI, pg/mL | 5.27 (5.07–5.90) | 5.53 (5.20–6.07) | 5.31 (4.97–5.91) | .416 | .662 | .282 | .489 |
| Log 10 HBD2, pg/mL | 3.81 (3.17–4.14) | 3.70 (3.27–4.30) | 3.87 (3.29–4.27) | .635 | .657 | .027 a | .030 a |
| Log 10 total protein, μg/mL | 2.35 (2.13–2.51) | 2.29 (2.10–2.48) | 2.25 (2.13–2.48) | .458 | .347 | N/A | N/A |
| IIIB inhibition, % | 53.52 (38.93–72.12) | 52.21 (33.89–70.31) | 53.77 (41.26–84.45) | .839 | .929 | .737 | .998 |
| BaL inhibition, % | 24.50 (6.59–31.11) | 28.64 (11.66–37.08) | 14.59 (–7.07 to 28.58) | .497 | .506 | .503 | .515 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


