Recognition of Clinical Features
Only a few infants are born in hospitals equipped for all eventualities. Infants with serious heart anomalies require transportation to a specialized pediatric cardiac center for detailed diagnostic assessment with echocardiography, and treatment that may include cardiac catheterization and/or surgery. Timely clinical recognition of the likely presence of a specific cardiac anomaly, that without intervention will result in serious deterioration of the baby’s condition (e.g., critical coarctation of the aorta, pulmonary valve atresia), is necessary for initiation of medical therapy to prevent and/or reverse clinical deterioration (e.g., administration of prostaglandin, inotropic agents, oxygen, and ventilation) and thereby provide the time and conditions necessary to transfer, evaluate, and treat the baby.
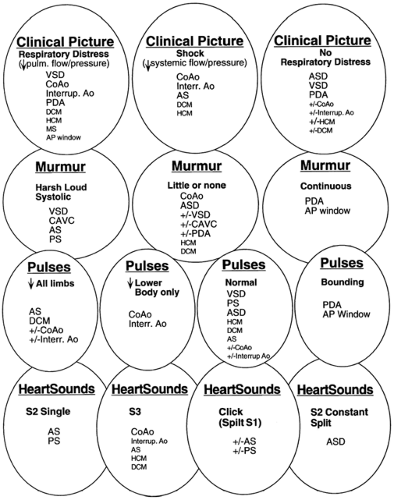
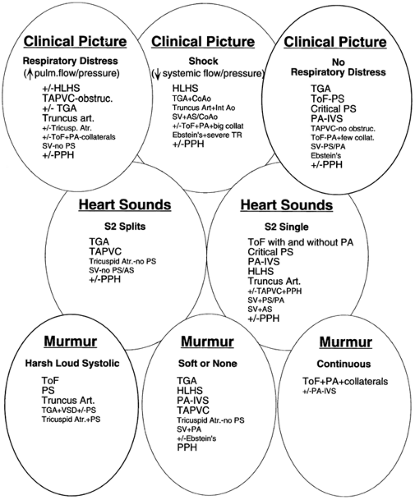
Initial evaluation includes assessment for cyanosis (See Color Plate) and of the infant’s well-being, perfusion, pulses and blood pressure in the extremities, respiratory work and rate, precordial activity, second heart sound splitting, and murmur intensity, quality, pitch, and timing. Chest radiograph and electrocardiogram (ECG) remain cost- and time-efficient tests that aid in the initial evaluation of suspected congenital heart disease. Any one of these alone is rarely diagnostic. A number of lesions result in cyanosis; quite a number of lesions are also associated with loud murmurs; others are associated with little or no murmur; some cause shock (see Fig. 33-7 and 33-8). Others have chest
radiographs with increased pulmonary arterial or venous markings, others have diminished pulmonary vascular markings. Most have an undistinguished electrocardiogram at birth, although some have left axis deviation on electrocardiogram (see Fig. 33-9 and 33-10). Most cardiac anomalies vary in their characteristics at presentation. Furthermore, often it is not possible to determine with certainty if the second heart sound is split or not, or if the pulmonary vascular markings on chest radiograph are normal vs. increased or normal versus decreased. Clinical analysis requires weighting of the categories of evidence regarding its certainty and other possibilities. A classical diagnostic approach based on sequential analysis of data categories is limited by these types of weakness in the clinical information and is no stronger than the weakest link in the chain of information. However, interweaving of the findings provides a matrix of diagnostic information that remains intact even when one category of findings is weak. Overlapping possible anomalies suggested from history, physical examination, chest radiograph and electrocardiogram, as if with a series of Venn diagrams (see Figs. 33-7, 33-8, 33-9 and 33-10), provides information that allows a careful observer to quickly determine which anomaly, or which two or three possible anomalies, is likely present. Comparing the anomalies consistent with the clinical presentation with the anomalies consistent with the murmur findings, other physical exam findings, chest radiograph findings, and electrocardiographic findings, usually focuses the list of possible anomalies on one or two primary choices (see Fig. 33-11). This may provide an important advantage in the timely and efficient management of potentially life threatening anomalies. For example, the combination of cyanosis, soft or no murmur, single S2, chest radiograph with decreased pulmonary vascular markings and normal heart size, and electrocardiogram R axis of 50° suggests pulmonary atresia, which is an anomaly in which life depends on maintaining ductal patency (see Fig. 33-8 and 33-10). Two-dimensional echocardiogram should be obtained promptly if significant cardiac disease is suspected. This technique when done by personnel trained for evaluation
of congenital cardiac anomalies in neonates accurately demonstrates the anatomy, occasionally uncovering a potentially lethal lesion before symptoms. Appropriate initial management (e.g., infusion of PGE1 in a cyanotic infant suspected to have pulmonary atresia) need not await availability of echocardiography (see Fig. 33-12 and Management Procedures for Severe Cardiac Disease).
radiographs with increased pulmonary arterial or venous markings, others have diminished pulmonary vascular markings. Most have an undistinguished electrocardiogram at birth, although some have left axis deviation on electrocardiogram (see Fig. 33-9 and 33-10). Most cardiac anomalies vary in their characteristics at presentation. Furthermore, often it is not possible to determine with certainty if the second heart sound is split or not, or if the pulmonary vascular markings on chest radiograph are normal vs. increased or normal versus decreased. Clinical analysis requires weighting of the categories of evidence regarding its certainty and other possibilities. A classical diagnostic approach based on sequential analysis of data categories is limited by these types of weakness in the clinical information and is no stronger than the weakest link in the chain of information. However, interweaving of the findings provides a matrix of diagnostic information that remains intact even when one category of findings is weak. Overlapping possible anomalies suggested from history, physical examination, chest radiograph and electrocardiogram, as if with a series of Venn diagrams (see Figs. 33-7, 33-8, 33-9 and 33-10), provides information that allows a careful observer to quickly determine which anomaly, or which two or three possible anomalies, is likely present. Comparing the anomalies consistent with the clinical presentation with the anomalies consistent with the murmur findings, other physical exam findings, chest radiograph findings, and electrocardiographic findings, usually focuses the list of possible anomalies on one or two primary choices (see Fig. 33-11). This may provide an important advantage in the timely and efficient management of potentially life threatening anomalies. For example, the combination of cyanosis, soft or no murmur, single S2, chest radiograph with decreased pulmonary vascular markings and normal heart size, and electrocardiogram R axis of 50° suggests pulmonary atresia, which is an anomaly in which life depends on maintaining ductal patency (see Fig. 33-8 and 33-10). Two-dimensional echocardiogram should be obtained promptly if significant cardiac disease is suspected. This technique when done by personnel trained for evaluation
of congenital cardiac anomalies in neonates accurately demonstrates the anatomy, occasionally uncovering a potentially lethal lesion before symptoms. Appropriate initial management (e.g., infusion of PGE1 in a cyanotic infant suspected to have pulmonary atresia) need not await availability of echocardiography (see Fig. 33-12 and Management Procedures for Severe Cardiac Disease).
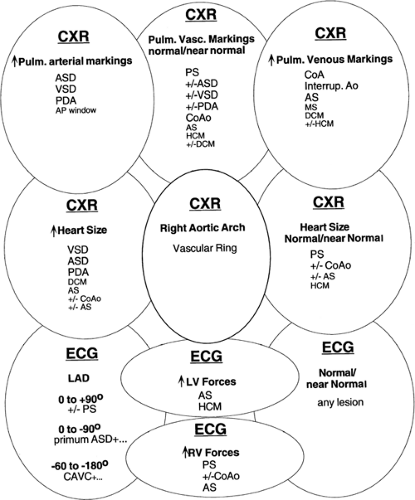 Figure 33-9 The differential diagnosis of chest radiographic and electrocardiographic findings in acyanotic neonates. Abbreviations, see Fig. 33-7; CXR, chest radiograph; LAD; left axis deviation; LV, left ventricle; Pulm. vasc. markings, pulmonary vascular markings; RV, right ventricle. |
Age of Presentation
Most children with critical congenital cardiac anomaly develop symptoms within the first weeks of life (8). The age when infants develop cardiac symptoms is diagnostically useful. For instance, although ventricular septal defect is far more common (Table 33-2), transposition of the great arteries, coarctation of the aorta, and the hypoplastic left heart syndrome are the most common life-threatening anomalies presenting in the first days of life (see Table 33-7). Isolated ventricular septal defect is not associated with cyanosis, the associated murmur generally develops after several days or more, and respiratory symptoms usually do not develop until after the first week of life. Among those whose problem is cyanosis, transposition of the great arteries is the leading cause through the third week of life; after that time, tetralogy of Fallot becomes the dominant cause of cyanosis. Among neonatal cardiac patients admitted because of respiratory symptoms, the hypoplastic left heart syndrome is the leading cause in the first week, complex coarctation leads in the second week, and thereafter, ventricular septal defect becomes the main cause (see Table 33-6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree